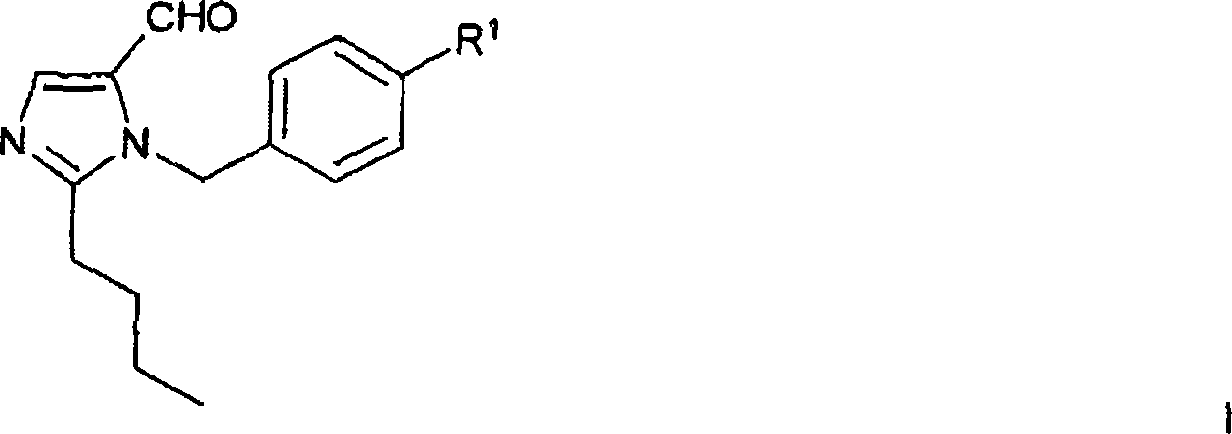
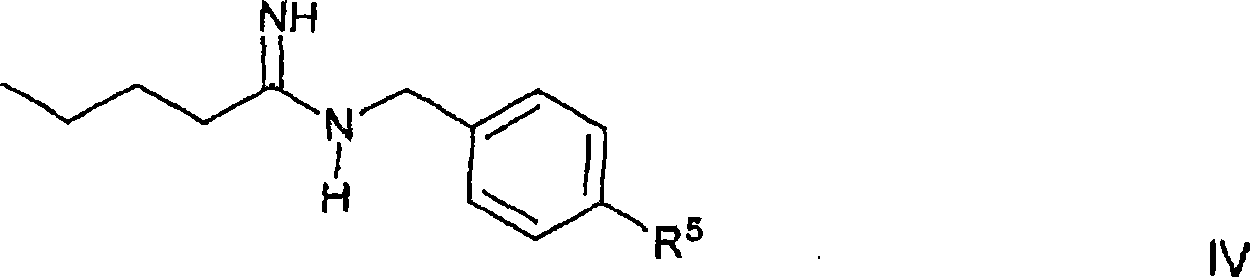
Method for the production of losartan
A general formula and compound technology, applied in the field of losartan production, can solve the problems of unreported precursor production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0198] Experimental part:
[0199] In the experimental part, the preparation of compounds appearing in Synthetic Schemes A, B or C is further described.
[0200] Experiment description
[0201] Equipment and Reagents
[0202] All dry solvents (CH 2 Cl 2 , THF, Et 2 (O, benzene, toluene, DMF, MeCN) were dried according to standard methods, such as removal of water and oxygen and distillation before use. React under an inert gas atmosphere (N 2 or Ar) and monitored by thin layer chromatography (TLC). Extraction solvents such as diethyl ether, ethyl acetate or chloroform. If not stated otherwise, the extracts were dried eg over anhydrous magnesium sulfate. If necessary, the reaction product is purified, for example, by column chromatography such as petroleum ether (60-90° C.) / ethyl acetate and chloroform / methanol as eluents. If model GF 254 The layer plate is used for thin layer chromatography, and the ethanol solution of iodine or phosphomolybdic acid (phosphormolybdn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com