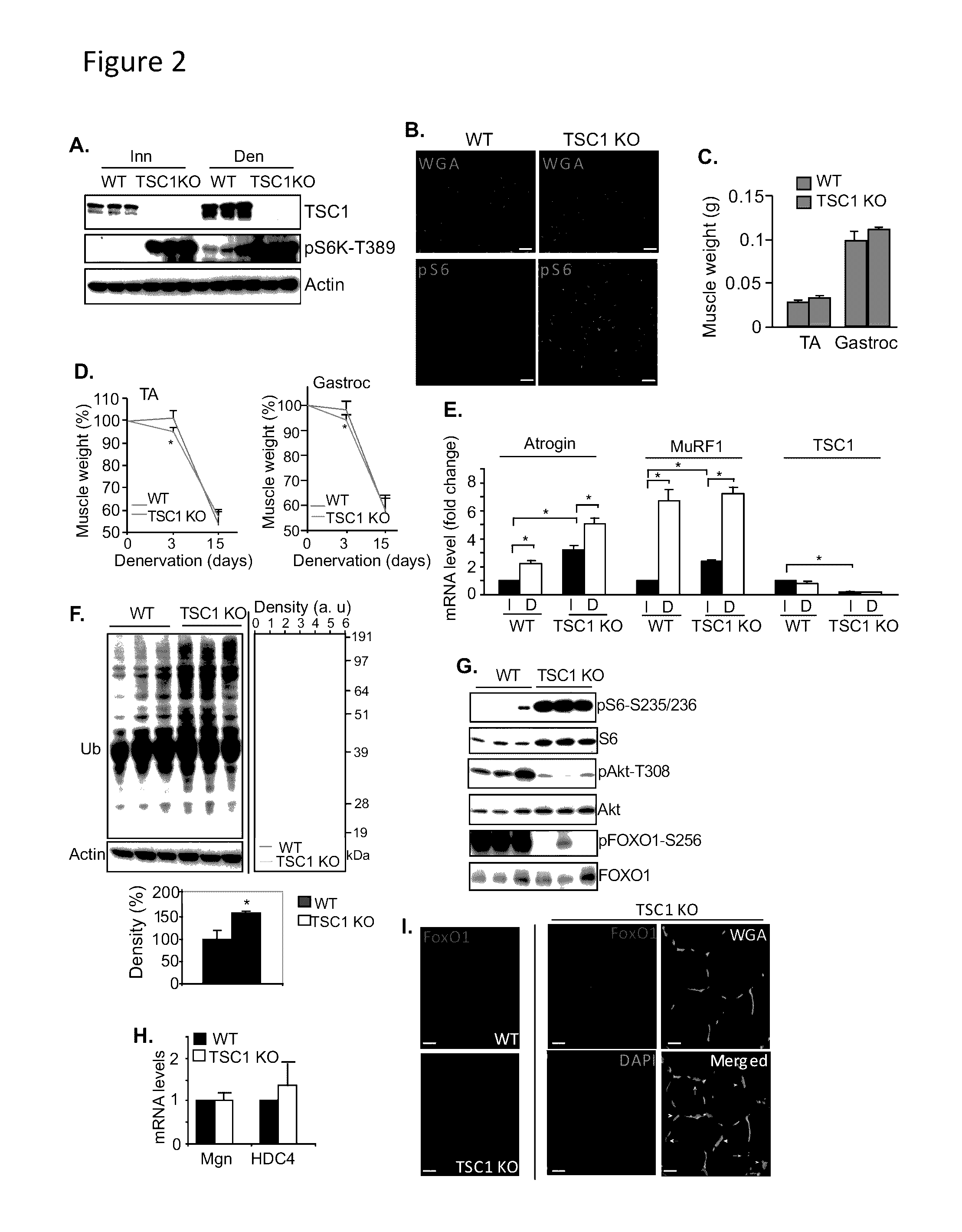
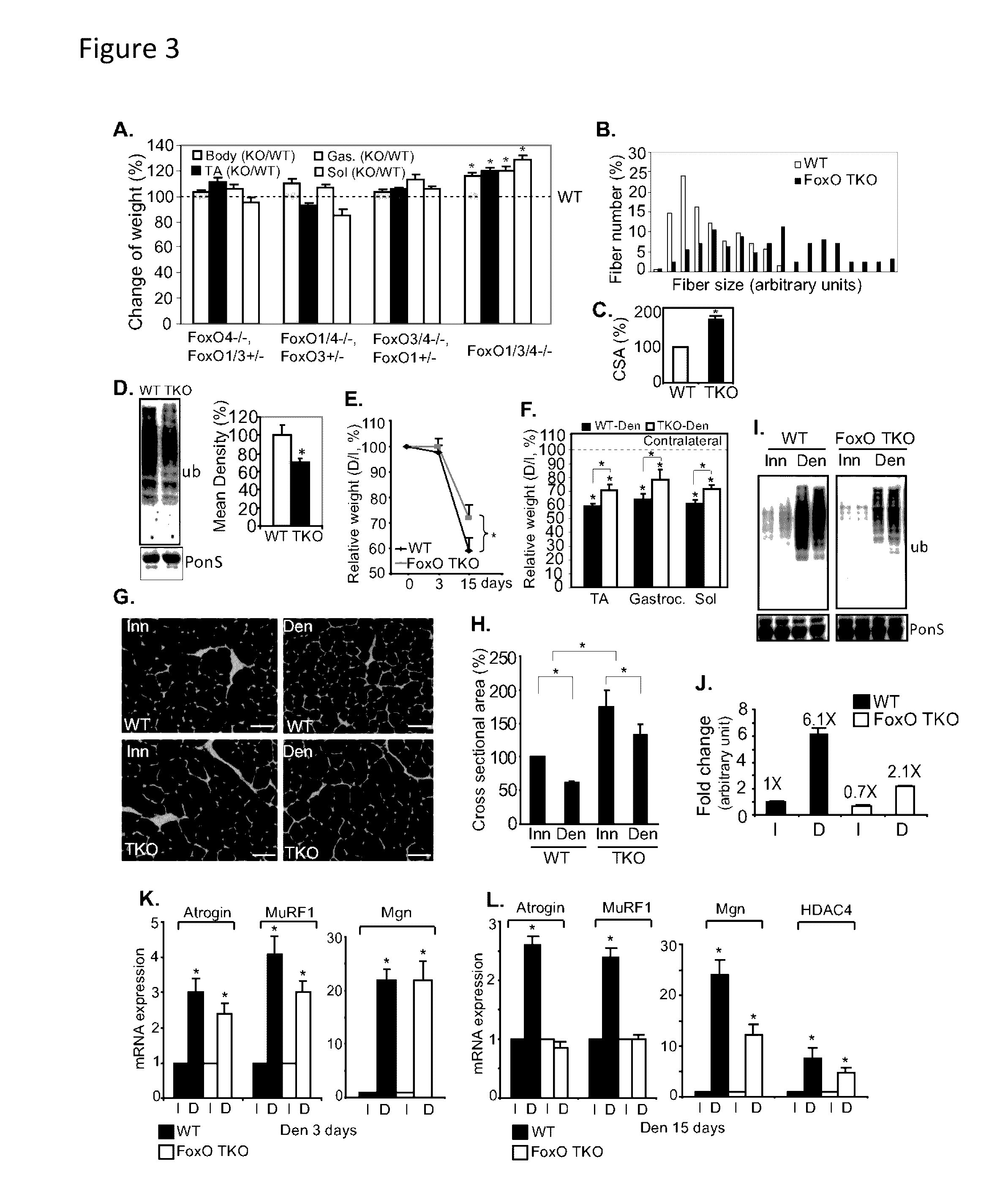
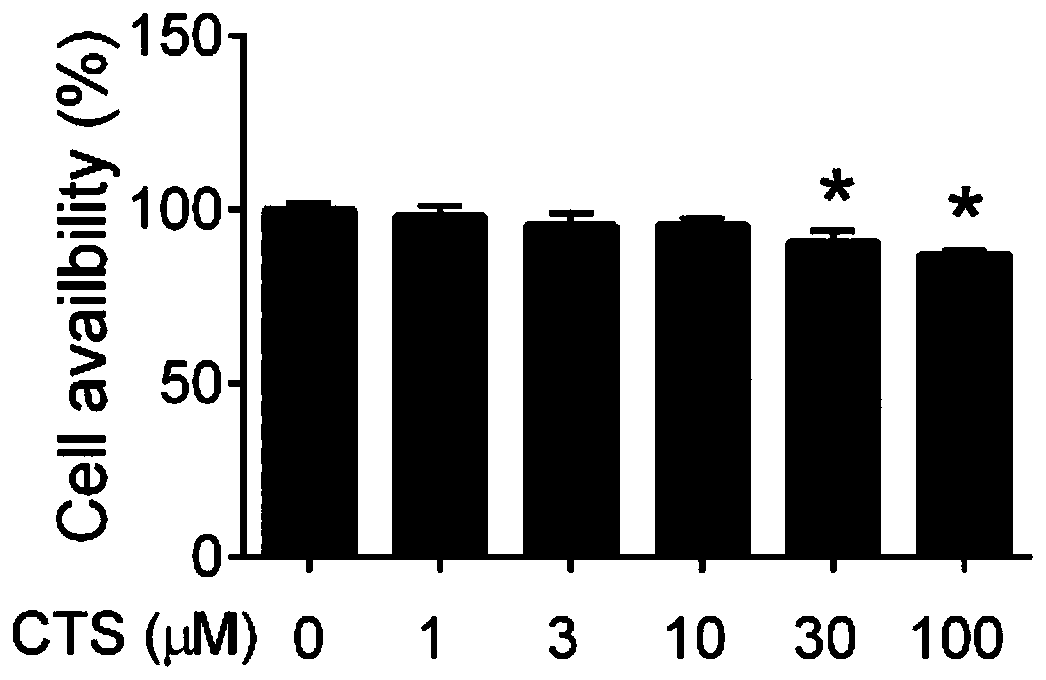
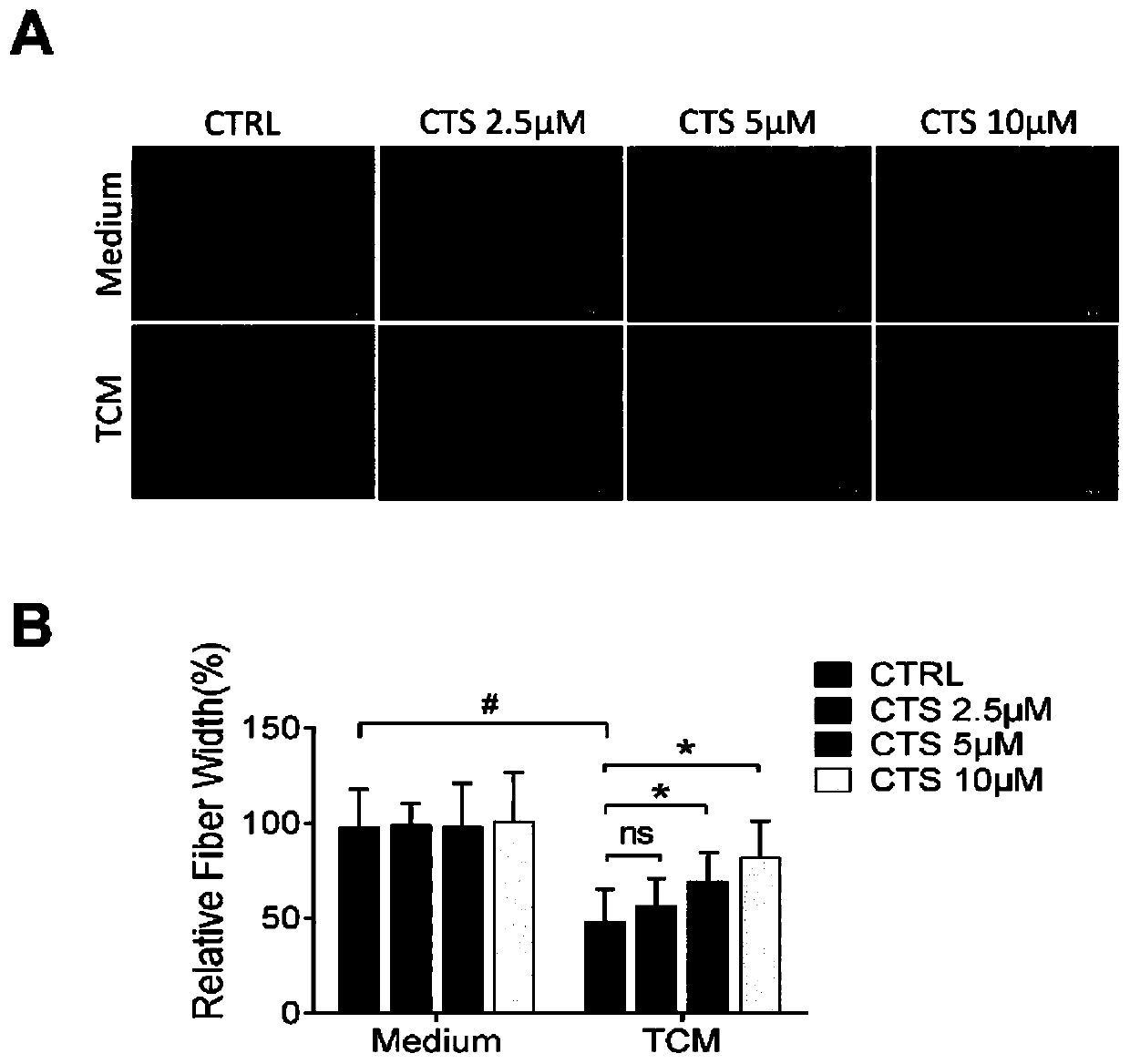
Patents
Literature
45 results about "Skeletal muscle atrophy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inhibitors of prolyl-hydroxylase-1 for the treatment of skeletal muscle degeneration
InactiveUS7858593B2Efficient transfectionImprovement in hybridization stabilityBiocideVirusesMuscle involvedSkeletal muscle atrophy
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +1
Thiol reactive agents as a therapeutic modality
InactiveUS20080145449A1Useful in treatmentImprove heart functionBiocideNervous disorderSkeletal muscle atrophyDisease
A patient with a disease associated with a receptor having a cysteine residue is treated with a thiol reactive agent. The diseases include neurodegenerative diseases. Diseases characterized by skeletal muscle atrophy are also treated.
Owner:DUKE UNIV
Inhibitors of prolyl-hydroxylase 1 for the treatment of skeletal muscle degeneration
InactiveUS20090047294A1Efficient transfectionImprovement in hybridization stabilityOrganic active ingredientsVirusesSkeletal muscle atrophyDisease
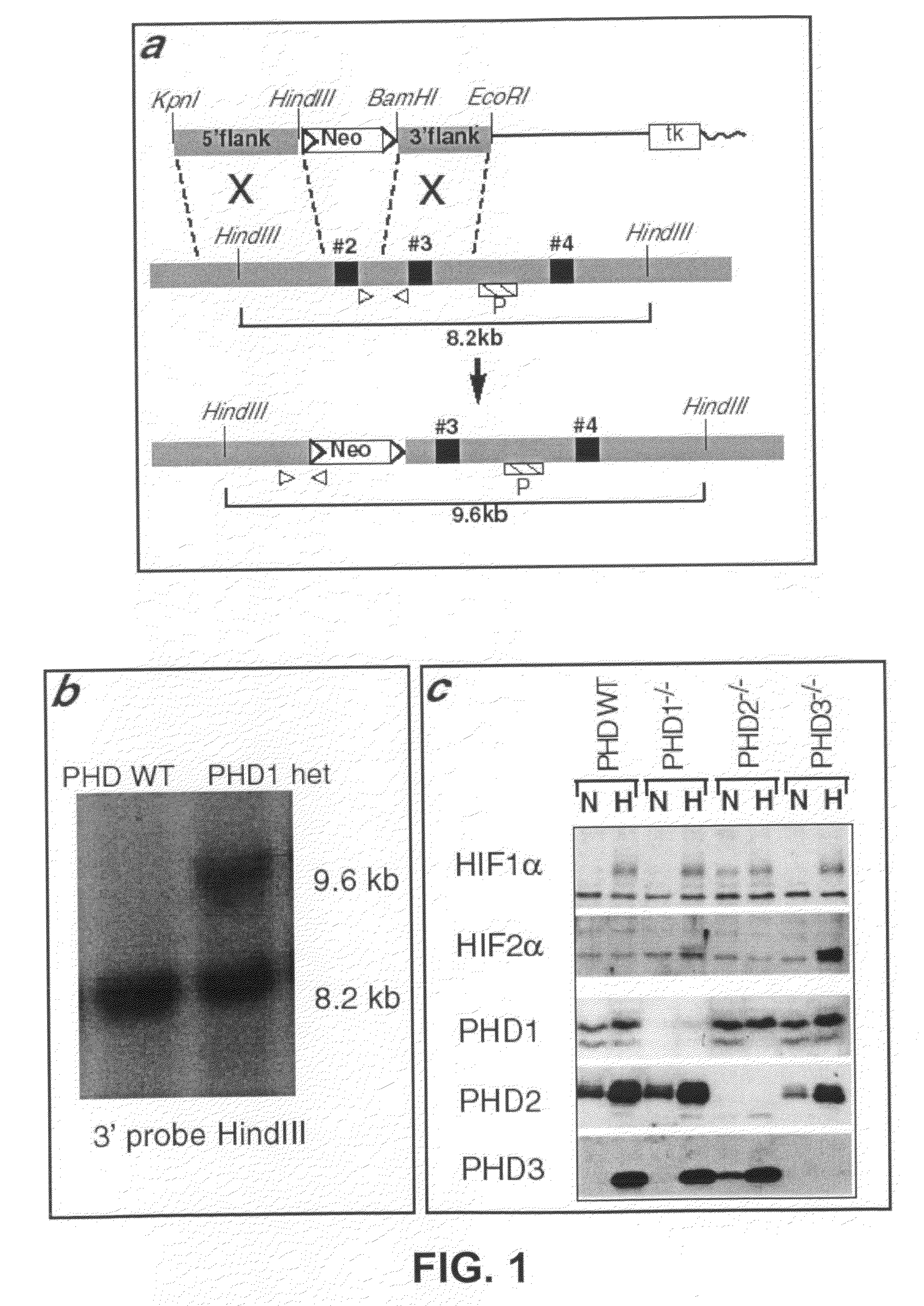
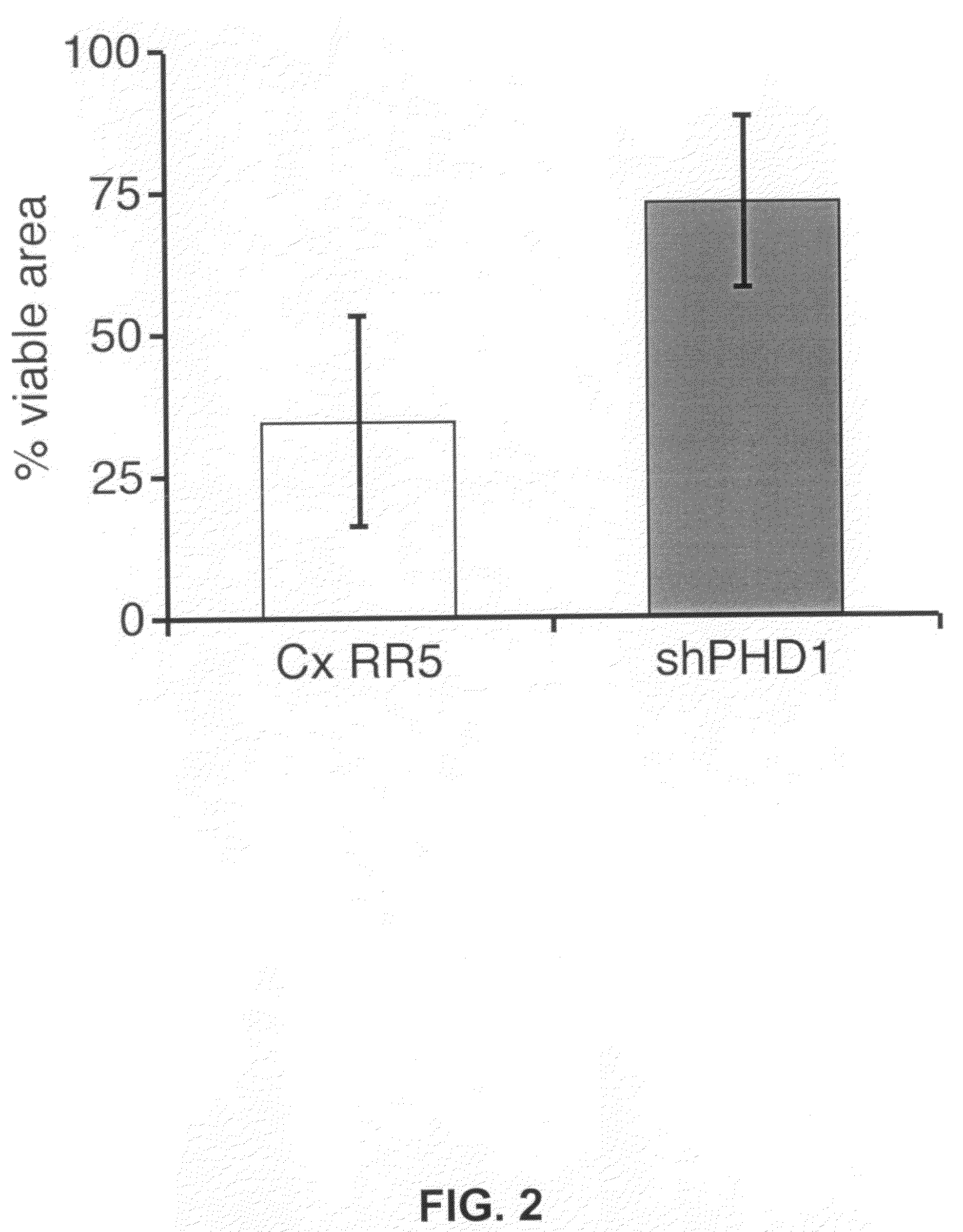
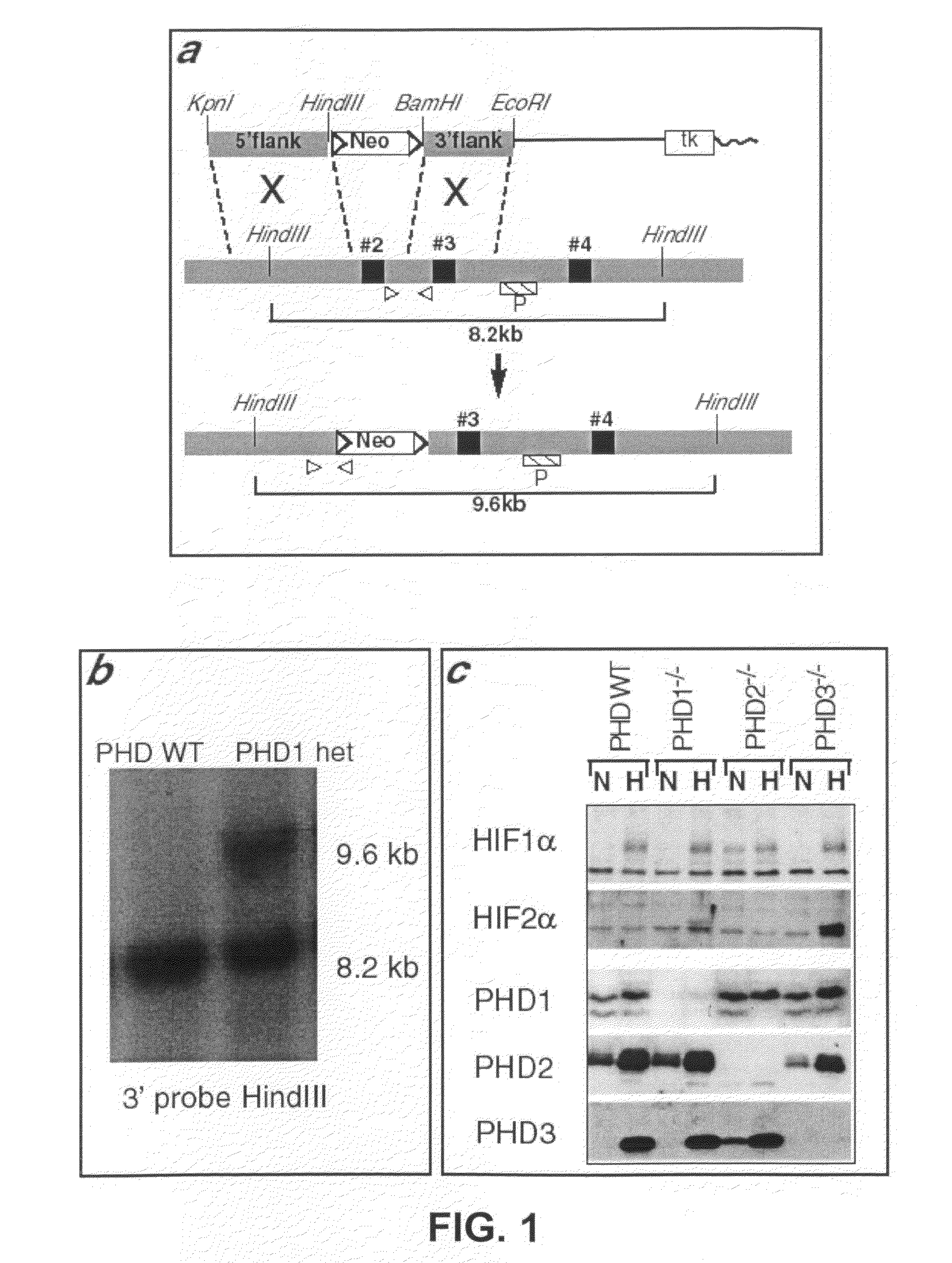
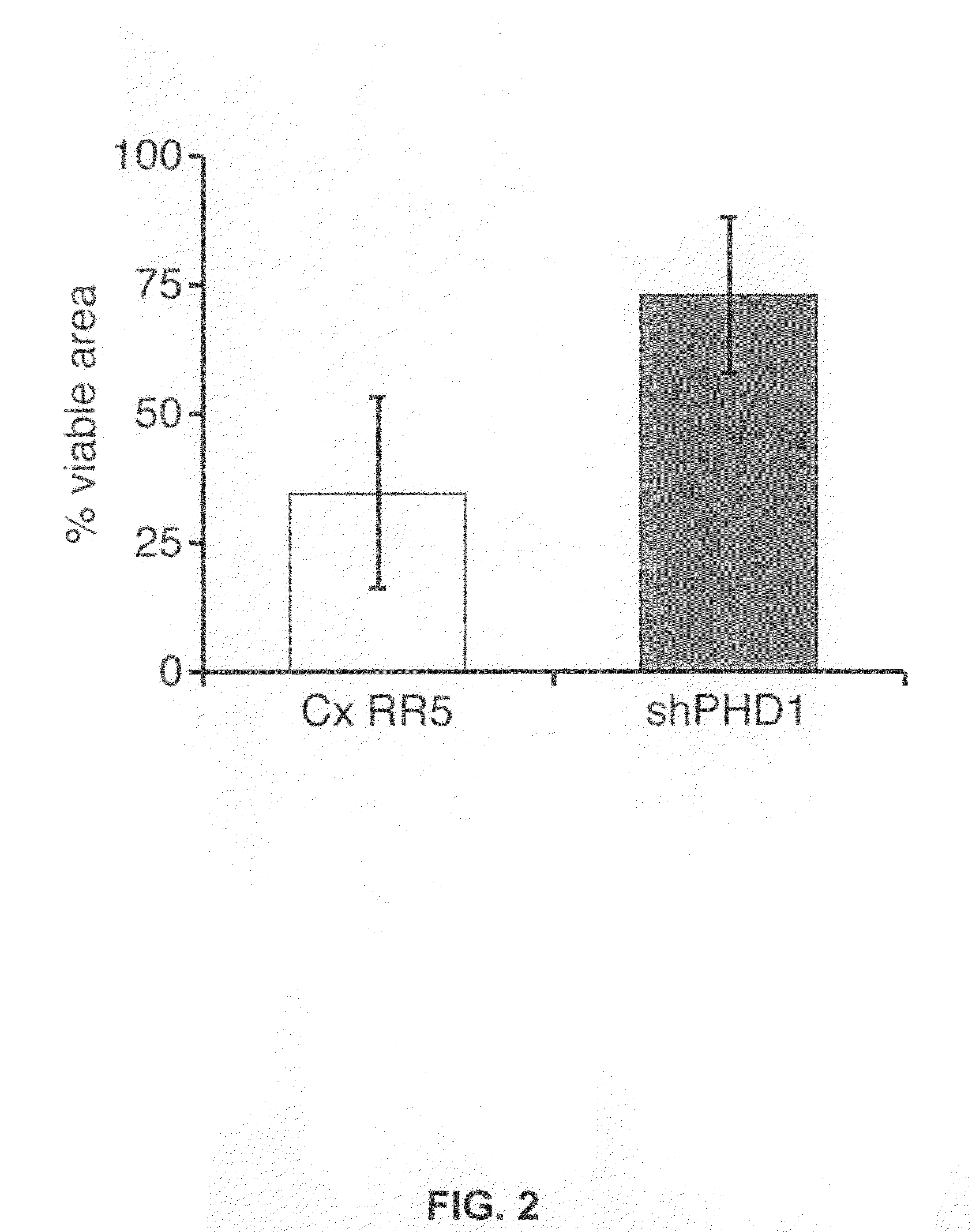
The invention relates to the field of muscle pathologies, more particularly to the field of diseases where skeletal muscle degeneration occurs. The invention describes transgenic mice that do not produce prolyl-hydroxylase-1, -2 or -3. It is revealed that the phenotype of the prolyl-hydroxylase 1 knock-out mouse is characterized by a protection of skeletal muscle atrophy due to a variety of muscle damages, especially ischemic insults. The invention thus relates to the use of molecules that can bind to prolyl-hydroxylase-1 for the prevention and / or treatment of skeletal muscle degeneration.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +1
Sesquiterpene lactone compounds as well as preparation method and application thereof
InactiveCN103641841AAvoid degradationImprove the level ofOrganic active ingredientsOrganic chemistryDrugSkeletal muscle atrophy
The invention provides a sesquiterpene lactone compound as well as a preparation method and application thereof, wherein the compound has a structure shown in a structural formula (I) or (II). The sesquiterpene lactone compound provided by the invention can be used as a drug for treating cachexia of human and animals, and is capable of treating the cachexia associated with various diseases. The sesquiterpene lactone compound is capable of effectively slowing down the loss of weight caused by skeletal muscle atrophy and fat degradation atrophy, obviously reducing tumor load, inhibiting the degradation of protein and increasing the levels of cell factors TNF-A and IL-6.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Flavonoids compound, preparation method and application thereof
InactiveCN104161767AInhibit the inflammatory responseAntibacterial agentsOrganic active ingredientsDiseaseProtein Degradations
The invention provides a flavonoids compound, a preparation method and an application of the flavonoids compound in medicine for prevention and treatment of cachexia. The compound has a structure shown in a structural formula (I) as follows. The provided flavonoids compound can be used for preparing the medicine for treating human and animal cachexia, and cachexia relative to a plurality of diseases can be treated, lowered body weight due to skeletal muscle atrophy and fat degradation atrophy can be effectively alleviated, tumour burden can be obviously mitigated, protein degradation is inhibited, and cytokine TNF-a and IL-6 level can be increased.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Methods for identifying compounds for regulating muscle mass or function using dopamine receptors
Owner:PROCTER & GAMBLE CO
Methods for identifying compounds for regulating muscle mass or function using corticotropin releasing factor receptors
InactiveUS20060198842A1Compounds screening/testingPeptide/protein ingredientsCorticotropinsScreening method
Screening methods for identifying compounds that bind to or activate corticotropin releasing factor2 receptors (CRF2R) and regulate or potentially regulate skeletal muscle mass or function in vivo are disclosed. Also disclosed are screening methods for identifying compounds that prolong or augment the activation of CRF2Rs or of CRF2R signal transduction pathways, increase CRF2R or increase CRF expression are provided. Pharmaceutical compositions comprising CRF2R agonists, antibodies to CRF2R and methods for increasing skeletal muscle mass or function or for the treatment of skeletal muscle atrophy using CRF2R as the target for intervention and methods for treatment of muscular dystrophies are described.
Owner:APTALIS PHARMA
CD29<+> human umbilical cord source mesenchymal stem cell and use thereof in preparation of drug for treating skeletal muscle atrophy in high-sugar and high-fat environments
ActiveCN107557332AImprove hyperlipidemiaImprove symptoms of skeletal muscle atrophyMetabolism disorderMuscular disorderAcute hyperglycaemiaAntigen
The invention discloses a CD29<+> human umbilical cord source mesenchymal stem cell and use thereof in the preparation of a drug for treating skeletal muscle atrophy in high-sugar and high-fat environments. The CD29<+> human umbilical cord source mesenchymal stem cell represents the following mesenchymal stem cell cytomembrane molecules: a human leukocyte differentiation antigen CD73, a human leukocyte differentiation antigen CD90, a human leukocyte differentiation antigen CD105 and a human leukocyte differentiation antigen CD29. According to the CD29<+> human umbilical cord source mesenchymalstem cell, the hyperlipidemia and hyperglycemia of a db<- / -> mouse can be obviously improved, muscle fiber cross sections of soleus and gastrocnemius muscle of the db<- / -> mouse can be increased, andthe contents and cell number of each myotube are increased, so that the symptoms of the skeletal muscle atrophy of the db<- / -> mouse are improved. According to the CD29<+> human umbilical cord sourcemesenchymal stem cell, the theoretical foundation and experiment basis are laid for the subsequent research and development of the drug for treating skeletal muscle atrophy in the high-sugar and high-fat environments, and the CD29<+> human umbilical cord source mesenchymal stem cell has wide application prospects.
Owner:JILIN TUO HUA BIOTECH
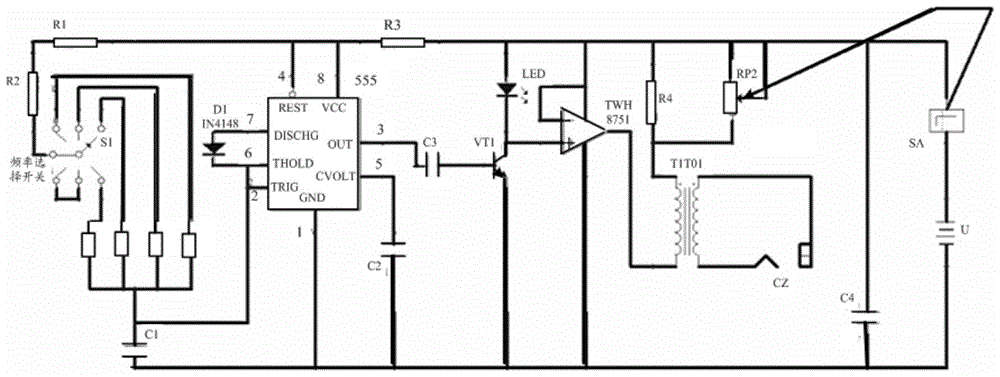
Circuit control device based on muscle vibration and vibrator
InactiveCN105708675AImprove practicalityMeet the preventionVibration massageSkeletal muscle atrophy555 timer IC
The invention provides a circuit control device and a vibrator based on muscle vibration. Wherein, the circuit control device includes: a multivibrator, the multivibrator includes a frequency adjustment switch, based on the 555 timer circuit, the frequency adjustment is realized by changing the resistance value of a plurality of resistors, and is used to adjust the above-mentioned multivibrator Transducers are capable of operating at several different frequencies. The invention solves the problem that the working frequency of the equipment used for muscle vibration training in the related art is relatively single and cannot adapt to the training needs of different parts, improves the practicability of vibration training, and can effectively meet the needs of skeletal muscles caused by long-term braking such as paralysis. Prevention of atrophy and skeletal muscle loss in aging.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
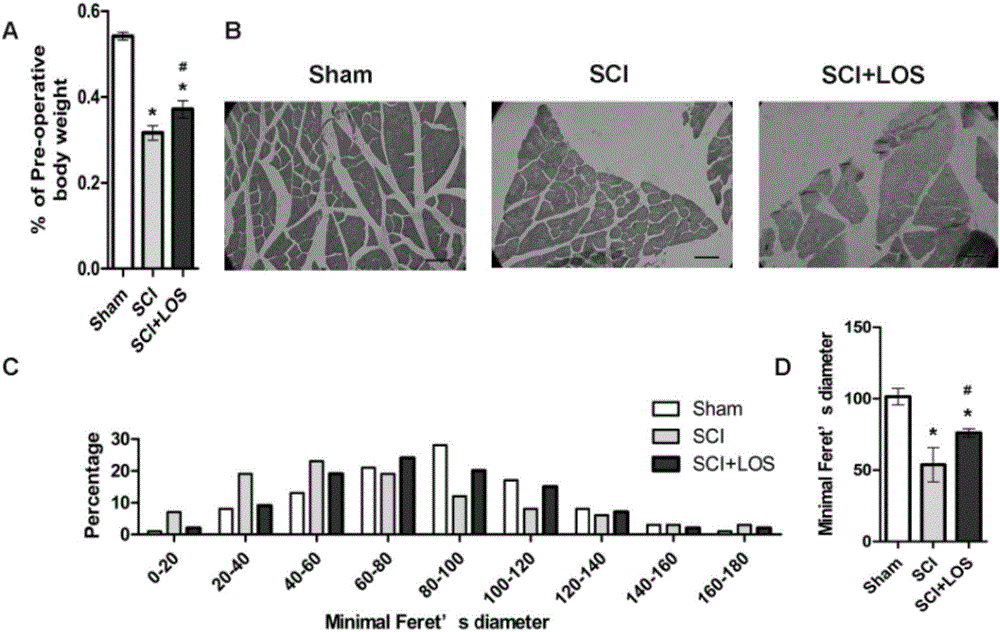
Medicine that cures muscle atrophy after spinal cord injury and its application method
InactiveCN106474119ARelieve atrophyPrevent atrophyOrganic active ingredientsNervous disorderSkeletal muscle atrophyPharmacon
The invention discloses a medicine that cures muscle atrophy after spinal cord injury and its application method: the medicine that cures muscle atrophy after spinal cord injury is losartan. The application method is that losartan activates a signal pathway of IGF1 / Akt / mTOR and inhibits an expression of MuRF-1; A receptor blocking pharmacon of angiotensin II takes effect through activating the pathway of IGF-1 / Art / mTOR and inhibiting a ubiquotin-proteasomes system. The receptor blocking pharmacon of angiotensin II can improve the skeletal muscle atrophy after spinal cord injury of rats and may take effect through activating the pathway of IGF-1 / Akt / mTOR and inhibiting the ubiquitin-proteasomes system, which provides a part of basis for later clinical application and combined therapy.
Owner:天津运三泽生物医药科技有限公司 +1
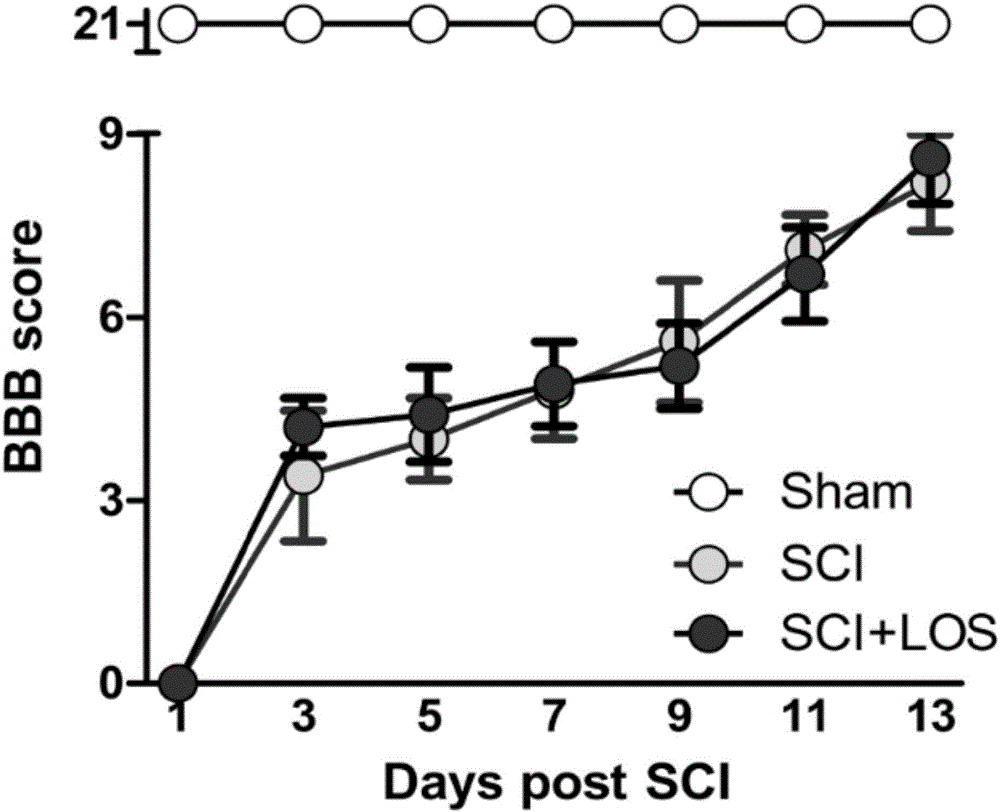
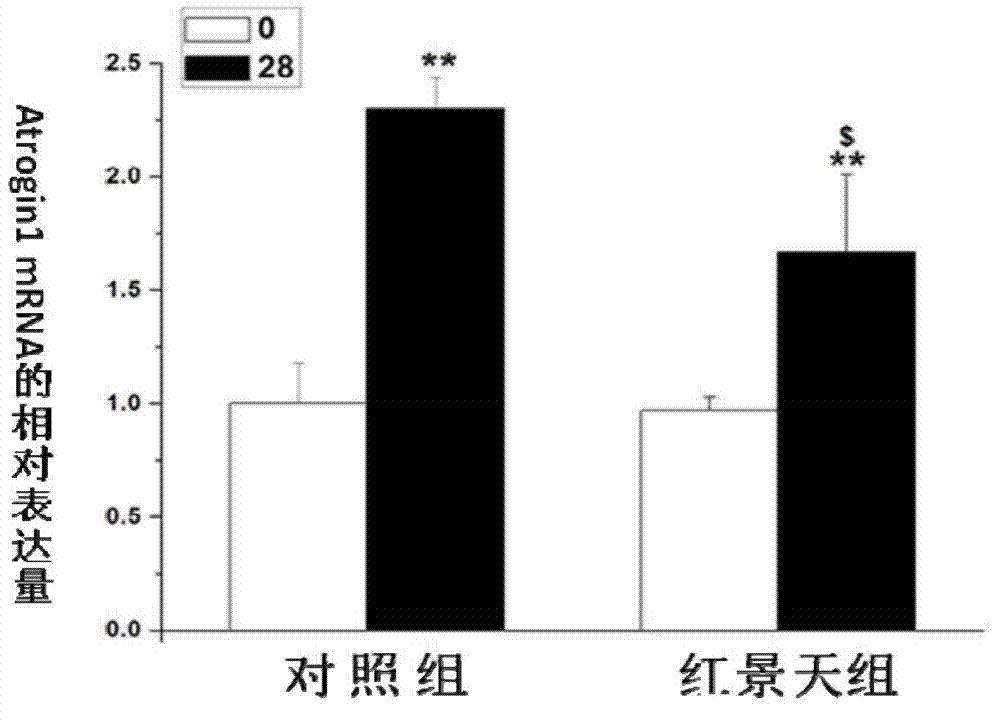
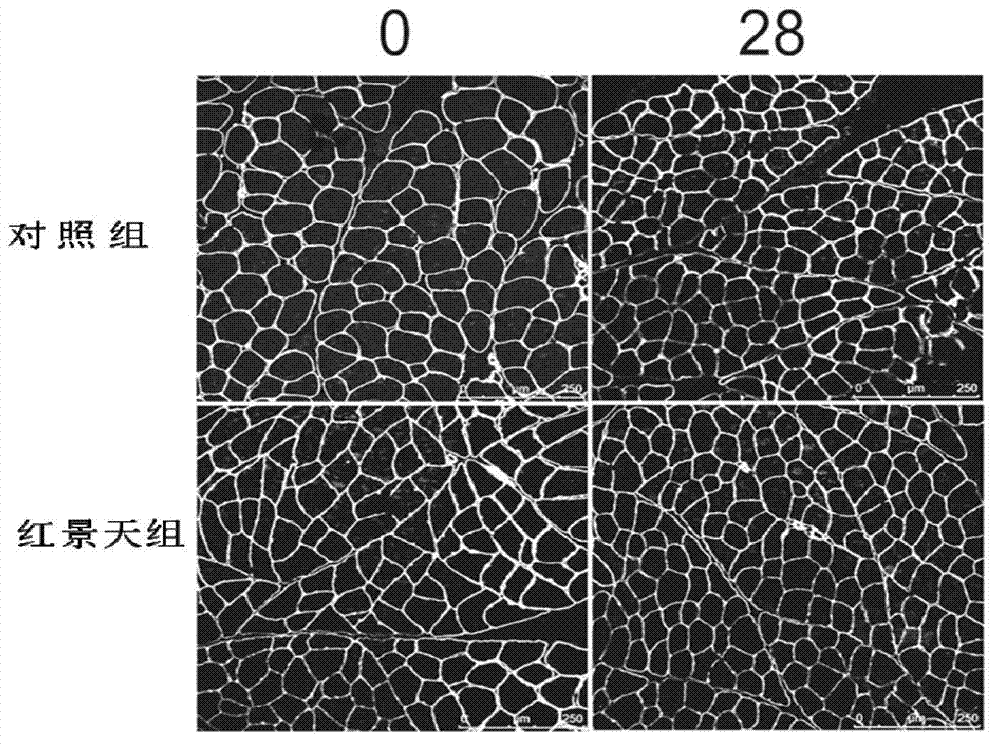
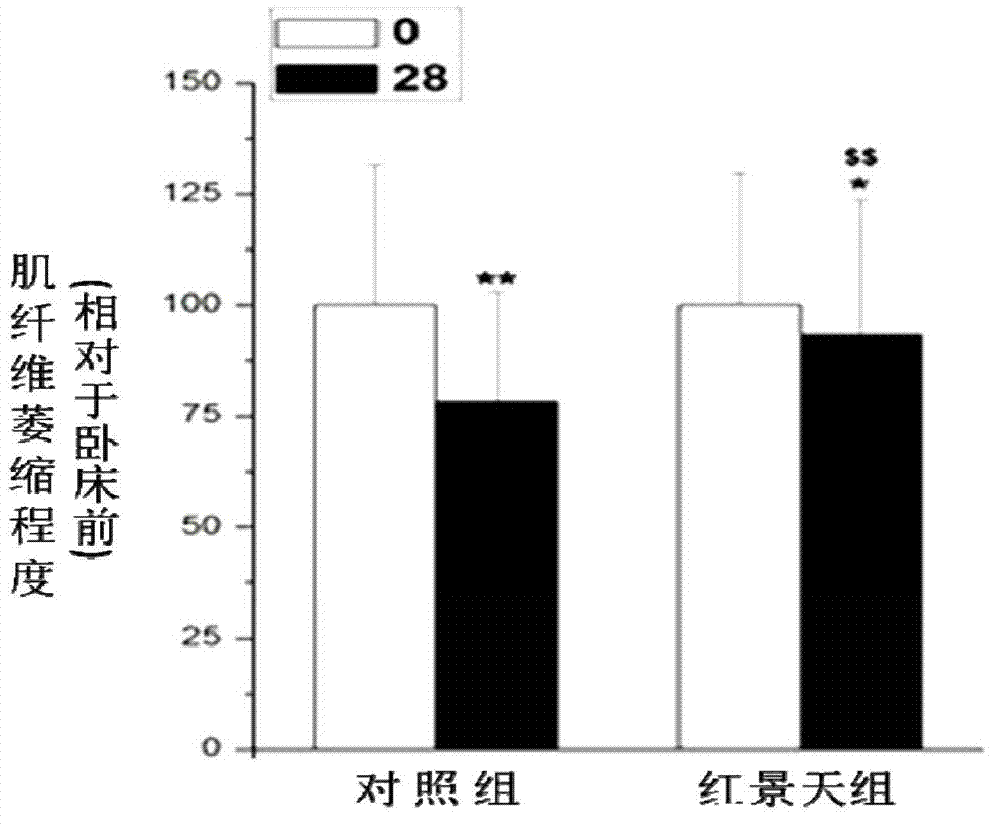
Application of rhodiola rosea in prevention and treatment of amyotrophy disease
ActiveCN102727560ADiscovery and demonstration of new capabilities in the treatment of muscle-wasting diseasesInhibition decreasedMuscular disorderNeuromuscular disorderDiseaseFiber
The invention relates to application of rhodiola rosea in prevention and treatment of an amyotrophy disease. An experiment shows that the rhodiola rosea can inhibit the expression of amyotrophy specific factor Atrogin-1 in the amyotrophy generating process and the reduction of the cross sectional area of skeletal muscle fiber, and can regulate the content of protein related to the muscle fiber in amyotrophy so that slow-twitch fibers protein can be increased, and fast-twitch fibers protein can be reduced, as a result, the generation of amyotrophy is resisted. According to the application, the rhodiola rosea serving as the active ingredient can be used for preparing the medicament for preventing and controlling a skeletal muscle atrophy disease.
Owner:SCI RES TRAINING CENT FOR CHINESE ASTRONAUTS
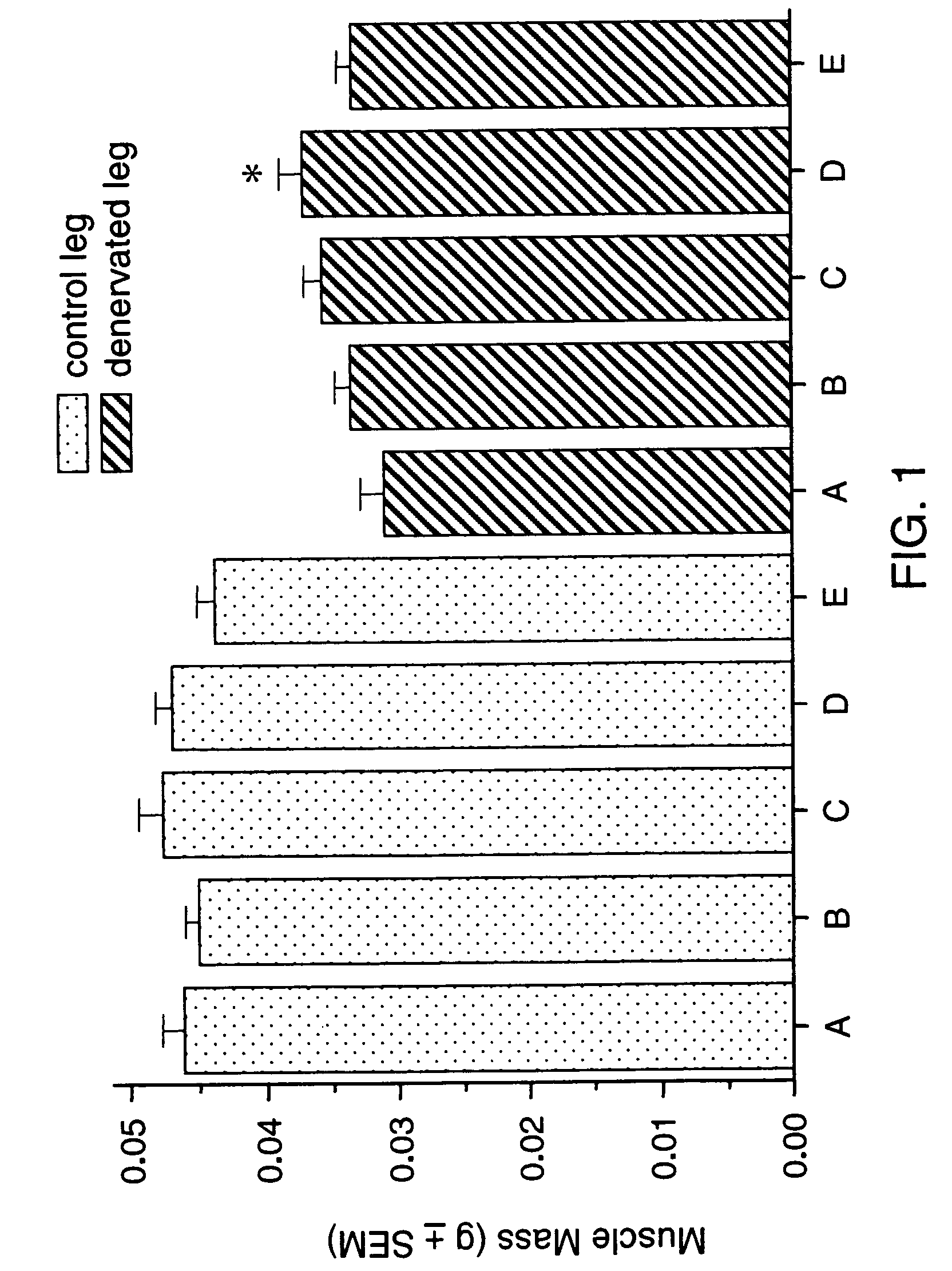
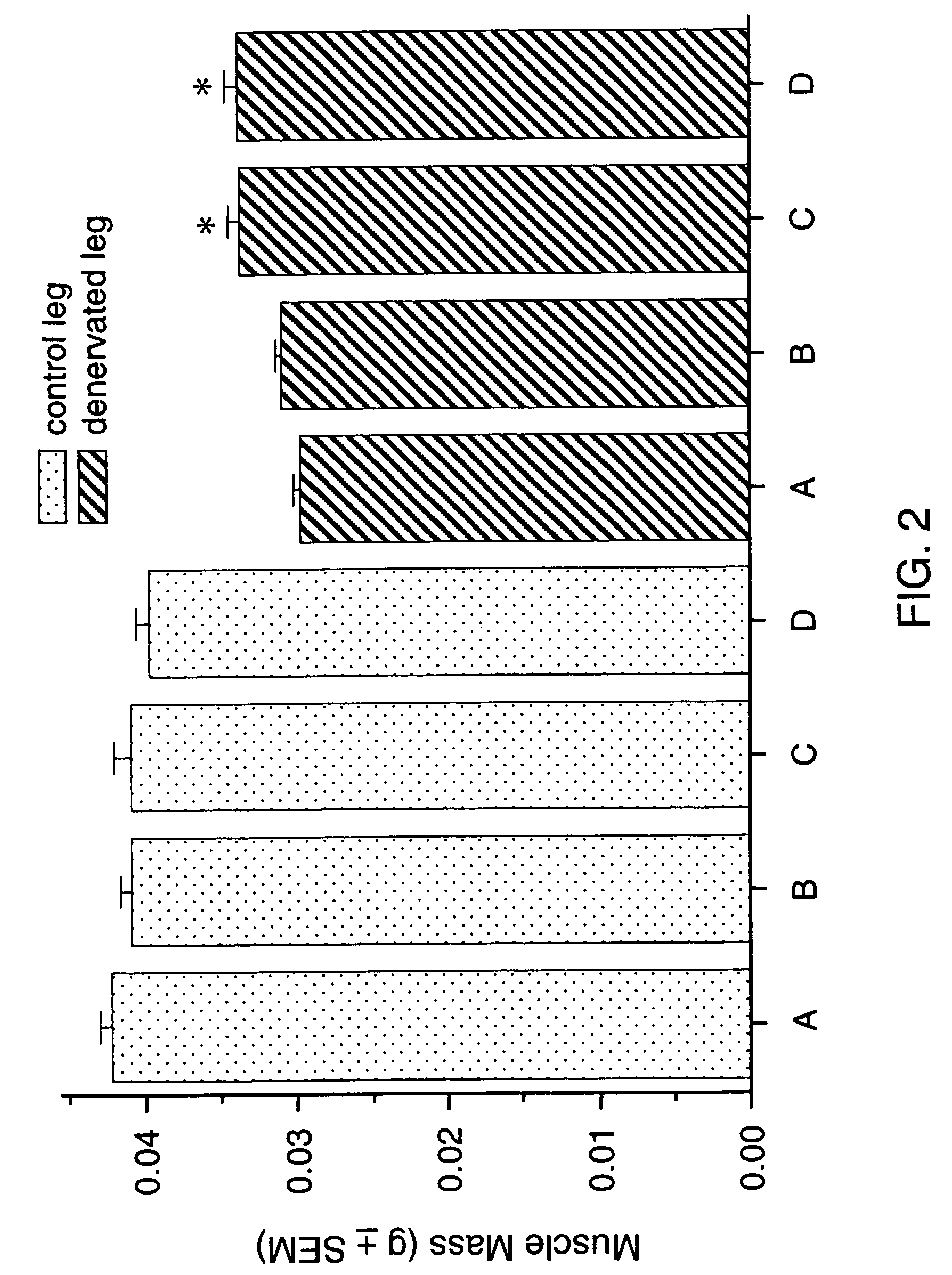
Methods for treating denervation-induced muscle atrophy
ActiveUS20140275228A1Reduced activityBiocideGenetic material ingredientsSkeletal muscle atrophyDenervation
The present invention provides methods for the treatment of denervation-induced skeletal muscle atrophy, and generally, denervation-induced skeletal muscle degeneration diseases using agents that decrease the activity of mammalian target of rapamycin and / or the activity of at least one of the Forkhead Box Transcription Factors 1, 3 and 4.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods for identifying compounds for regulating muscle mass or function using corticotropin releasing factor receptors
Screening methods for identifying compounds that bind to or activate corticotropin releasing factor2 receptors (CRF2R) and regulate or potentially regulate skeletal muscle mass or function in vivo are disclosed. Also disclosed are screening methods for identifying compounds that prolong or augment the activation of CRF2Rs or of CRF2R signal transduction pathways, increase CRF2R or increase CRF expression are provided. Pharmaceutical compositions comprising CRF2R agonists, antibodies to CRF2R and methods for increasing skeletal muscle mass or function or for the treatment of skeletal muscle atrophy using CRF2R as the target for intervention and methods for treatment of muscular dystrophies are described.
Owner:APTALIS PHARMA
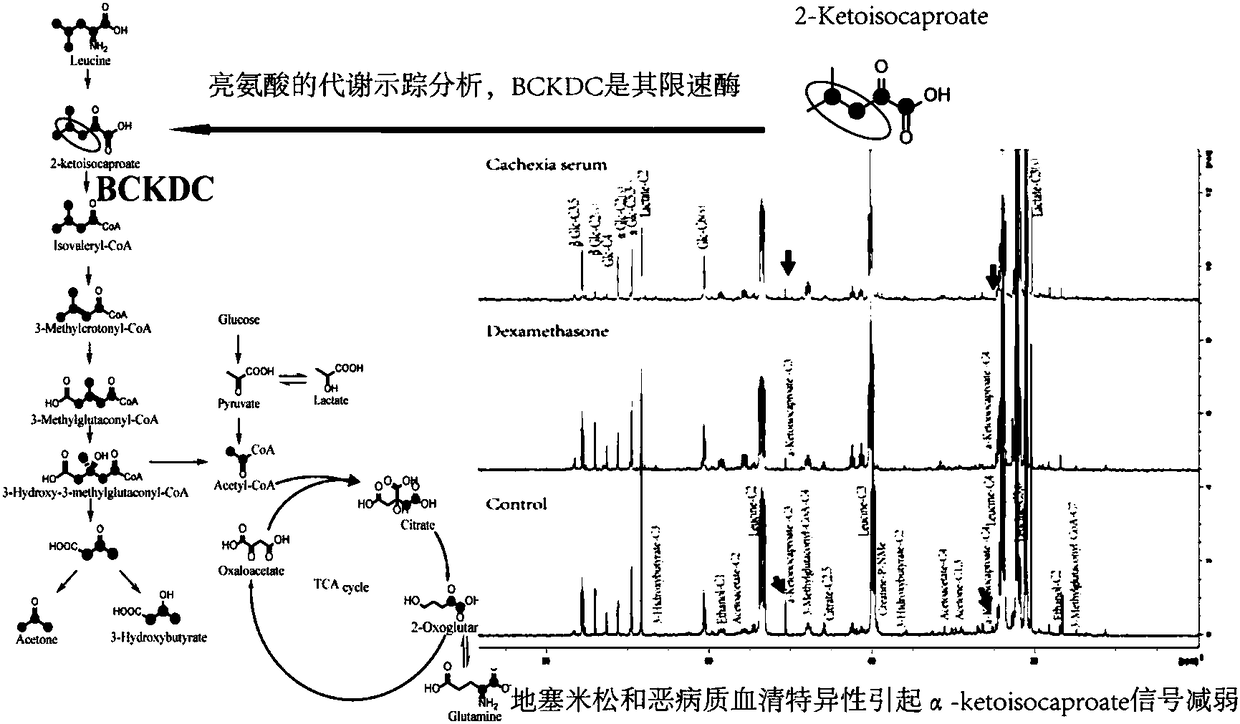
Therapeutic drug target for preventing and treating skeletal muscle atrophy and application of therapeutic drug target
PendingCN108498798AMicrobiological testing/measurementBiological material analysisMetabolic enzymesDrug target
The invention relates to a therapeutic drug target for preventing and treating skeletal muscle atrophy and an application of the therapeutic drug target, and provides the application of a BCKDC (branched-chain alpha-ketoacid dehydrogenase complex) or BCKDK (branched-chain alpha-ketoacid dehydrogenase kinase) as the therapeutic drug target to prevention or treatment of skeletal muscle atrophy and an application of the BCKDC or BCKDK to preparation of drugs for preventing or treating skeletal muscle atrophy and particularly provides a method for screening skeletal muscle atrophy resistant drugsin vivo and in vitro. According to the method, the BCKDC or a gene of the BCKDC is taken as a drug action object or BCKDK or a gene of BCKDK is taken as the drug action object, and an inhibitor of theBCKDC or an activator of BCKDK is selected as a primarily screened drug candidate for preventing or treating skeletal muscle atrophy. The key restrictive metabolic enzyme regulation mechanism of thetherapeutic drug target is analyzed from the perspective of tumor cachexia metabolism regulation, the drug intervention target is determined, the therapeutic drug target has potential clinical application value in treatment of skeletal muscle atrophy, and a new platform is provided for screening of the drugs for treating skeletal muscle atrophy.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Methods for identifying compounds for regulating muscle mass or function using dopamine receptors
Screening methods for identifying compounds that bind to or activate (D1 or D5 dopamine receptors individually or in combination) or potentially regulate skeletal muscle mass or function in vivo. Also disclosed are screening methods for identifying compounds that prolong or augment the activation of D1 or D5 dopamine receptors or of D1 or D5 dopamine receptor signal transduction pathways and increase D1 or D5 dopamine receptor expression. Pharmaceutical compositions comprising D1 or D5 dopamine receptor agonists, antibodies to D1 or D5 dopamine receptors and methods for increasing skeletal muscle mass or function or for the treatment of skeletal muscle atrophy using D1 or D5 dopamine receptors as the target for intervention and methods for treatment of muscular dystrophies are described.
Owner:THE PROCTER & GAMBLE COMPANY
Medical application of enzymatic hydrolysate of icariin and main component baohuoside I of icariin
InactiveCN112138017ALow effective dosePlay a protective effectOrganic active ingredientsMuscular disorderSkeletal muscle atrophyHydrolysate
The invention discloses a medical application of an enzymatic hydrolysate of icariin and a main component baohuoside I of the icariin, and particularly relates to an application of the enzymatic hydrolysate of icariin and the main component baohuoside I of the icariin in preparing a medicine for treating skeletal muscle atrophy. In-vitro and in-vivo experiments prove that the enzymatic hydrolysateof icariin or baohuoside I can significantly reduce the skeletal muscle atrophy degree, including increase of muscle fiber cross-sectional area, gastrocnemius muscle wet weight / body mass ratio and aerobic exercise ability, and can up-regulate skeletal muscle protein content by regulating an autophagy system, thus having great application prospects.
Owner:SHANGHAI UNIV OF T C M
Neurotrypsin inhibitors
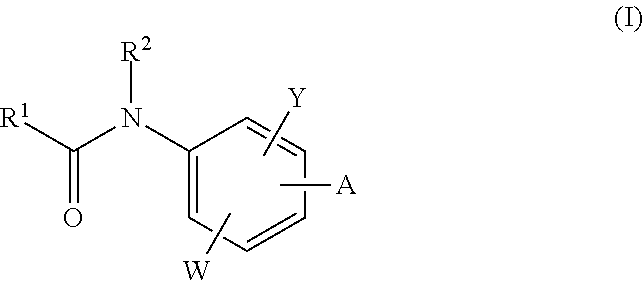
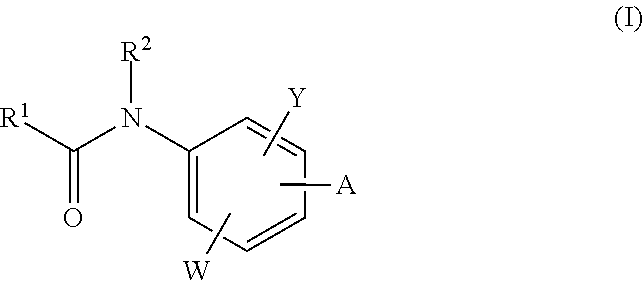
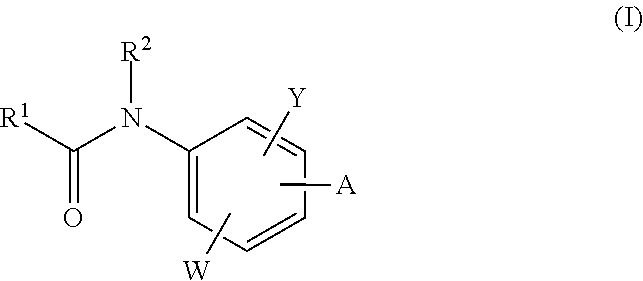
The invention relates to acylamino-phthalic acid amides and related compounds of formula (I) wherein A is —CON—R3R4, —NR5COR6, —NHR7, —OR8, —SR9, —CH2NR10R11, —(CH2)2-R12, —CH═CH—R12, —C≡C—R12, optionally substituted phenyl, optionally substituted thiophenyl, or optionally substituted 1,2,3-triazol-4-yl, W is hydrogen, hydroxy or carboxymethoxy, Y is carboxy, methoxycarbonyl or 2H-tetrazol-5-yl, and the various substituents R have the meanings indicated in the description. These compounds are useful for the treatment and / or prophylaxis of skeletal muscle atrophy, schizophrenia and Alzheimer's disease, and as cognitive enhancers.
Owner:NEUROTUNE AG
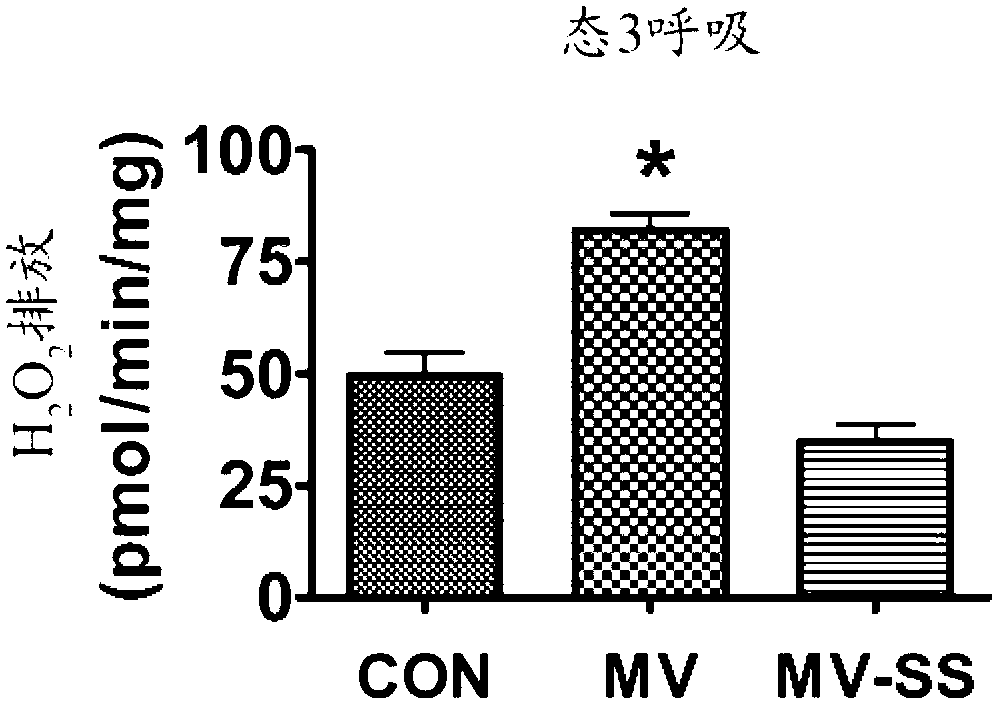
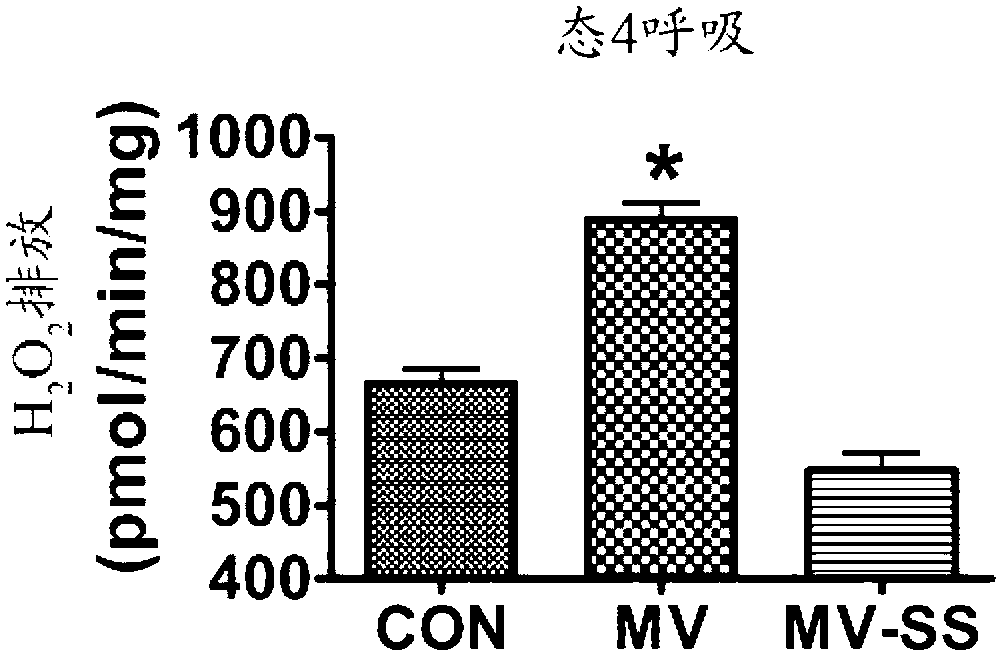
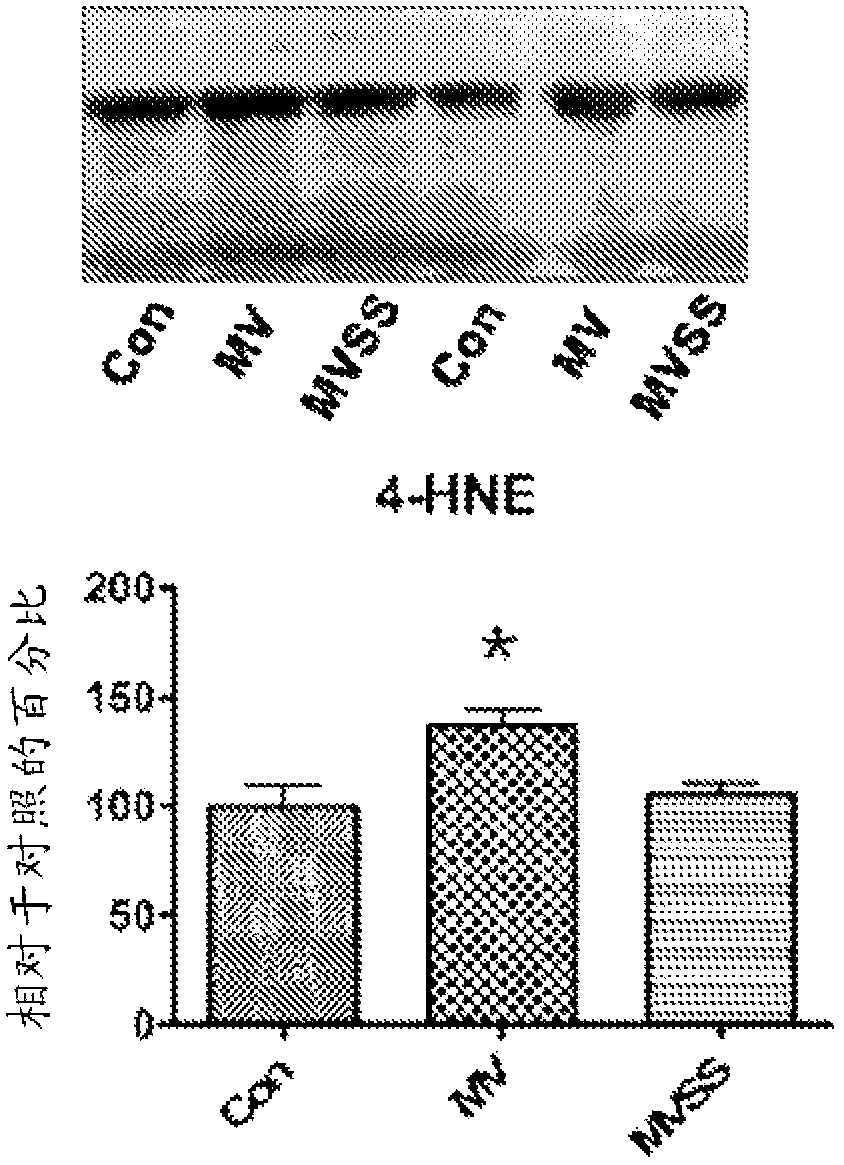
Mitochondrial-targeted antioxidants protect against mechanical ventilation-induced diaphragm dysfunction and skeletal muscle atrophy
The present disclosure provides methods and compositions for preventing or treating MV-induced or disuse-induced skeletal muscle infirmities in a mammalian subject. The methods further include administering to the subject an effective amount of an aromatic-cationic peptide.
Owner:UNIV OF FLORIDA RES FOUNDATION INC +1
Methods for identifying compounds for regulating muscle mass or function using dopamine receptors
Screening methods for identifying compounds that bind to or activate (D1 or D5 dopamine receptors individually or in combination) or regulate or potentially regulate skeletal muscle mass or function in vivo. Also disclosed are screening methods for identifying compounds that prolong or augment the activation of D1 or D5 dopamine receptors or of D1 or D5 dopamine receptor signal transduction pathways and increase D1 or D5 dopamine receptor expression. Pharmaceutical compositions comprising D1 or D5 dopamine receptor agonists, antibodies to D1 or D5 dopamine receptors and methods for increasing skeletal muscle mass or function or for the treatment of skeletal muscle atrophy using D1 or D5 dopamine receptors as the target for intervention and methods for treatment of muscular dystrophies are described.
Owner:THE PROCTER & GAMBLE COMPANY
Methods for treating denervation-induced muscle atrophy
The present invention provides methods for the treatment of denervation-induced skeletal muscle atrophy, and generally, denervation-induced skeletal muscle degeneration diseases using agents that decrease the activity of mammalian target of rapamycin and / or the activity of at least one of the Forkhead Box Transcription Factors 1, 3 and 4.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Application of cryptotanshinone in preparing medicine for preventing and treating cachexia skeletal muscle atrophy
InactiveCN111166754AAtrophy improvementAvoid degradationOrganic active ingredientsMuscular disorderSkeletal muscle atrophyAtrophy
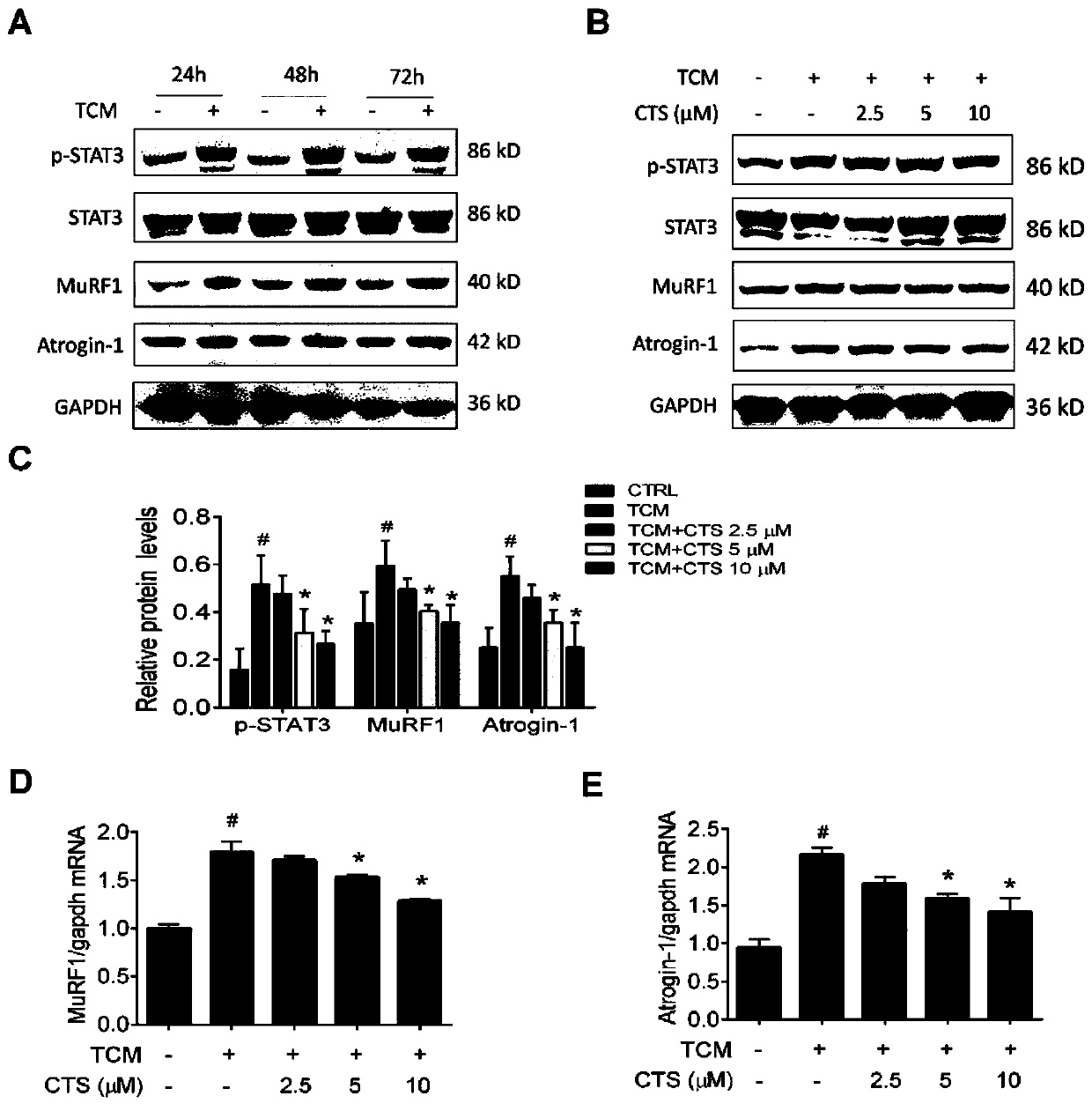
The invention provides application of cryptotanshinone in preparing a medicine for preventing and treating cachexia skeletal muscle atrophy. The medicine comprises an active component of cryptotanshinone and further comprises pharmaceutically acceptable auxiliary materials. Cryptotanshinone is capable of alleviating C2C12 myotube atrophy, and in addition, remarkably reducing expression of proteinsp-STAT3, MuRF1 and Atrogin-1 in muscles to inhibit degradation of skeletal muscle proteins, and novel ideas are provided for preparing medicines for treating and preventing cachexia skeletal muscle atrophy.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
Alk5 inhibitors as skeletal muscle hypertrophy inducers
The present invention relates to ALK5 inhibitors and their uses as skeletal muscle hypertrophy inducers as well as to promote skeletal muscle regeneration, to prevent skeletal muscle atrophy, or in the treatment or prevention of a disease or injury resulting in loss of skeletal muscle tissue and / or muscle weakness. It further relates to the non-therapeutic use of such compounds to increase musclemass, muscle strength and / or muscle performance in a subject.
Owner:赛途公司
Methods for identifying compounds for regulating muscle mass or function using corticotropin releasing factor receptors
Screening methods for identifying compounds that bind to or activate corticotropin releasing factor2 receptors (CRF2R) and regulate or potentially regulate skeletal muscle mass or function in vivo are disclosed. Also disclosed are screening methods for identifying compounds that prolong or augment the activation of CRF2Rs or of CRF2R signal transduction pathways, increase CRF2R or increase CRF expression are provided. Pharmaceutical compositions comprising CRF2R agonists, antibodies to CRF2R and methods for increasing skeletal muscle mass or function or for the treatment of skeletal muscle atrophy using CRF2R as the target for intervention and methods for treatment of muscular dystrophies are described.
Owner:APTALIS PHARMA
Inhibitors Of Neurotrypsin
InactiveUS20090170950A1Treatment and/or prophylaxis of diseasesTreatment and/or prophylaxis of skeletal muscle atrophyBiocideCompound screeningNEUROTRYPSINDisease cause
The invention relates to a method for determining whether a compound is a neurotrypsin inhibitor, characterized in that the compound is incubated together with neurotrypsin, a variant thereof or a fragment comprising the protease domain and with a protein or peptide comprising agrin, a variant thereof or a fragment comprising the α- or the β-cleavage site of agrin, in an aqueous buffer solution, and the amount of cleavage of agrin is measured. Additionally, the invention relates to inhibitors of neurotrypsin found by this method, in particular to compounds of formulawherein Hal1 and Hal2 are fluorine, chlorine or bromine, and the use of such inhibitors for the treatment and / or prophylaxis of diseases caused by deficiency of synapses, for example skeletal muscle atrophy, schizophrenia, and cognitive disturbance.
Owner:UNIV ZURICH +1
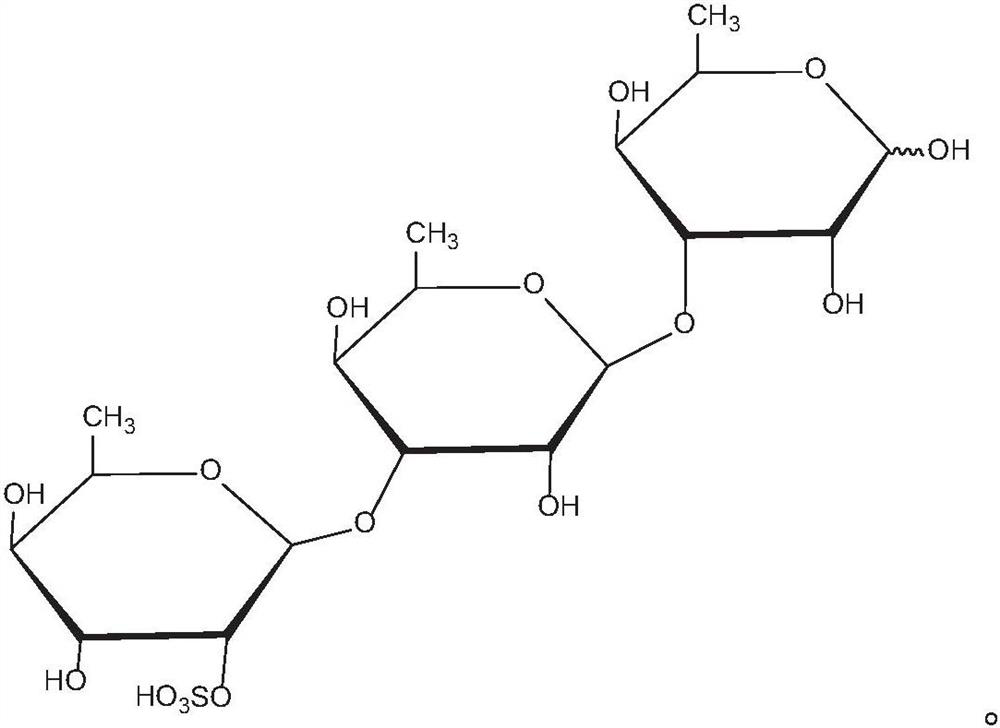
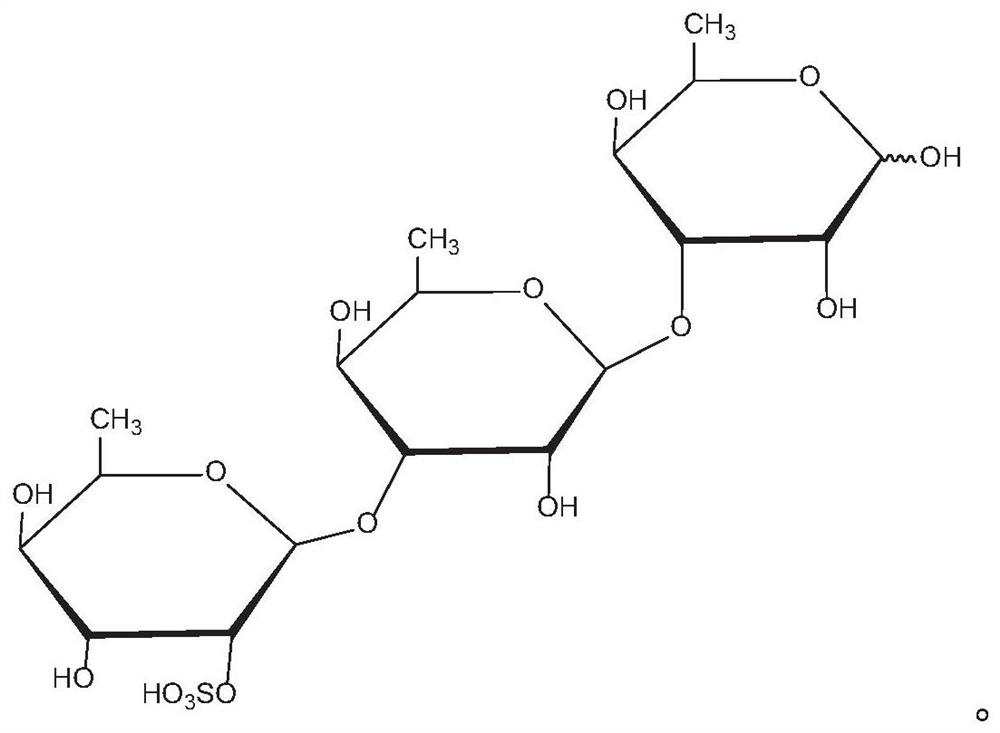
Application of rhamnose monosulfate and derivative thereof in resisting skeletal muscle atrophy
PendingCN111632057AInhibition of differentiationPromote productionEsterified saccharide compoundsOrganic active ingredientsSkeletal muscle atrophyMyosin
The embodiment of the invention provides an application of rhamnose monosulfate and a derivative thereof in resisting skeletal muscle atrophy. The rhamnose monosulfate or the rhamnose monosulfate derivative is applied to a medicine for resisting skeletal muscle atrophy, or the rhamnose monosulfate or the rhamnose monosulfate derivative is applied to a functional food for resisting skeletal muscleatrophy. The rhamnose monosulfate is used for inhibiting differentiation inhibition of TNF-alpha on skeletal muscle cells C2C12, promoting generation of myosin MHC and myoblast myogenin of C2C12, andpromoting conversion of skeletal muscle myocytes into muscle fibers.
Owner:QINGDAO CENT HOSPITAL
cd29 + Human umbilical cord-derived mesenchymal stem cells and their use in the preparation of drugs for the treatment of skeletal muscle atrophy in high-sugar and high-fat environments
ActiveCN107557332BImprove hyperlipidemiaImprove symptoms of skeletal muscle atrophyMetabolism disorderMuscular disorderWhite blood cellHUMAN LEUKOCYTE DIFFERENTIATION ANTIGEN
The invention discloses a CD29<+> human umbilical cord source mesenchymal stem cell and use thereof in the preparation of a drug for treating skeletal muscle atrophy in high-sugar and high-fat environments. The CD29<+> human umbilical cord source mesenchymal stem cell represents the following mesenchymal stem cell cytomembrane molecules: a human leukocyte differentiation antigen CD73, a human leukocyte differentiation antigen CD90, a human leukocyte differentiation antigen CD105 and a human leukocyte differentiation antigen CD29. According to the CD29<+> human umbilical cord source mesenchymalstem cell, the hyperlipidemia and hyperglycemia of a db<- / -> mouse can be obviously improved, muscle fiber cross sections of soleus and gastrocnemius muscle of the db<- / -> mouse can be increased, andthe contents and cell number of each myotube are increased, so that the symptoms of the skeletal muscle atrophy of the db<- / -> mouse are improved. According to the CD29<+> human umbilical cord sourcemesenchymal stem cell, the theoretical foundation and experiment basis are laid for the subsequent research and development of the drug for treating skeletal muscle atrophy in the high-sugar and high-fat environments, and the CD29<+> human umbilical cord source mesenchymal stem cell has wide application prospects.
Owner:JILIN TUO HUA BIOTECH
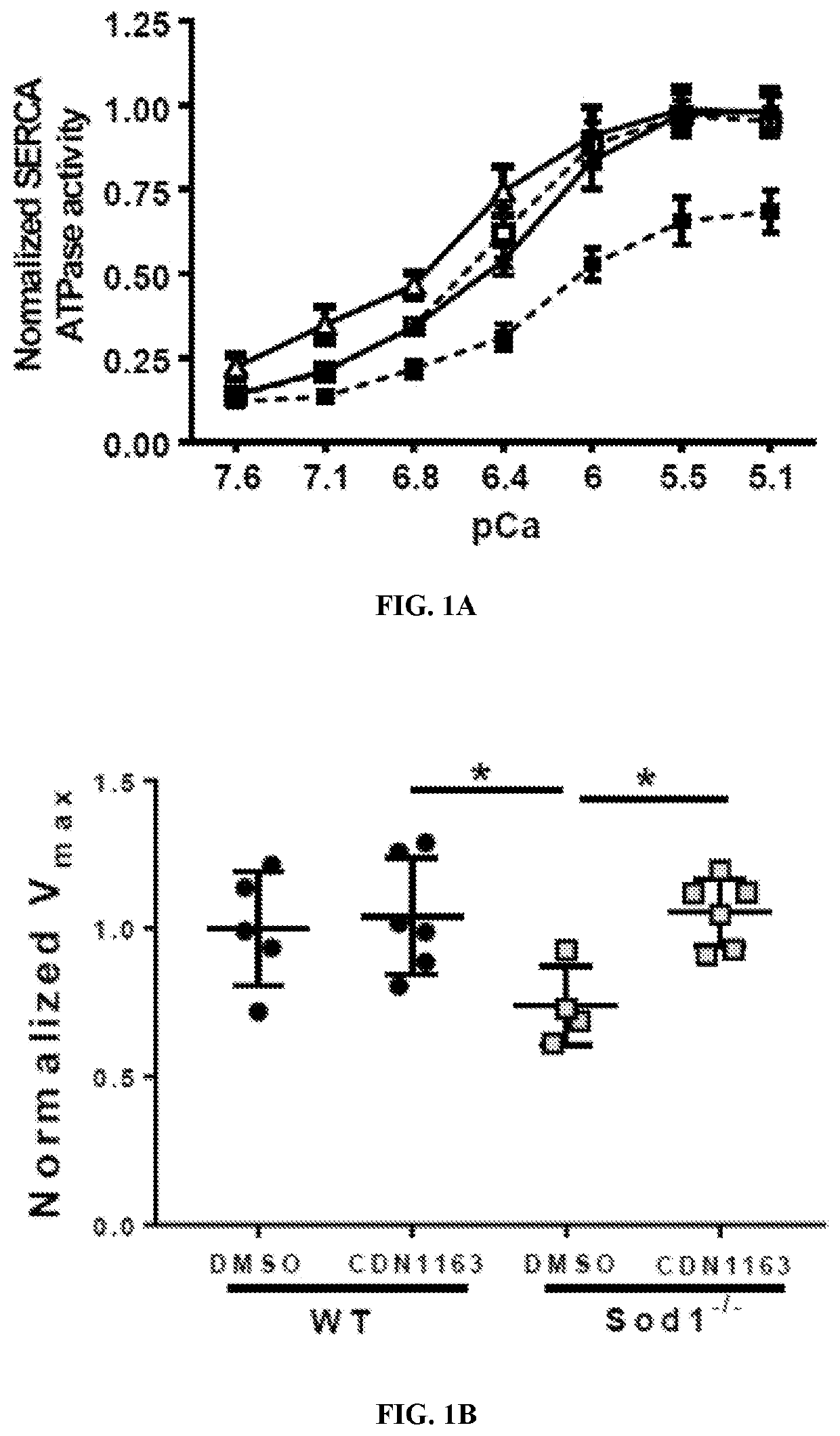
Treatment for age- and oxidative stress-associated muscle atrophy and weakness
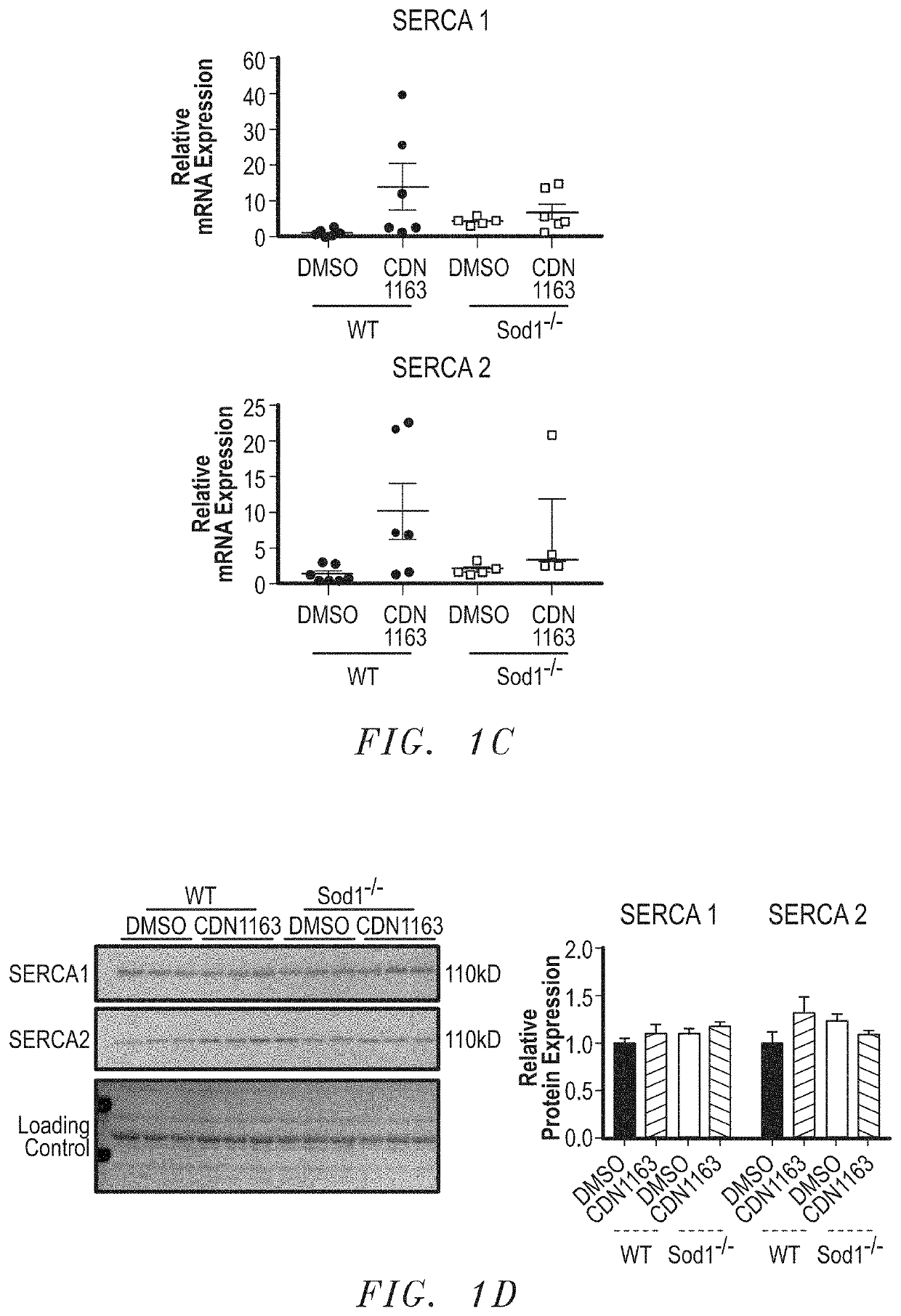
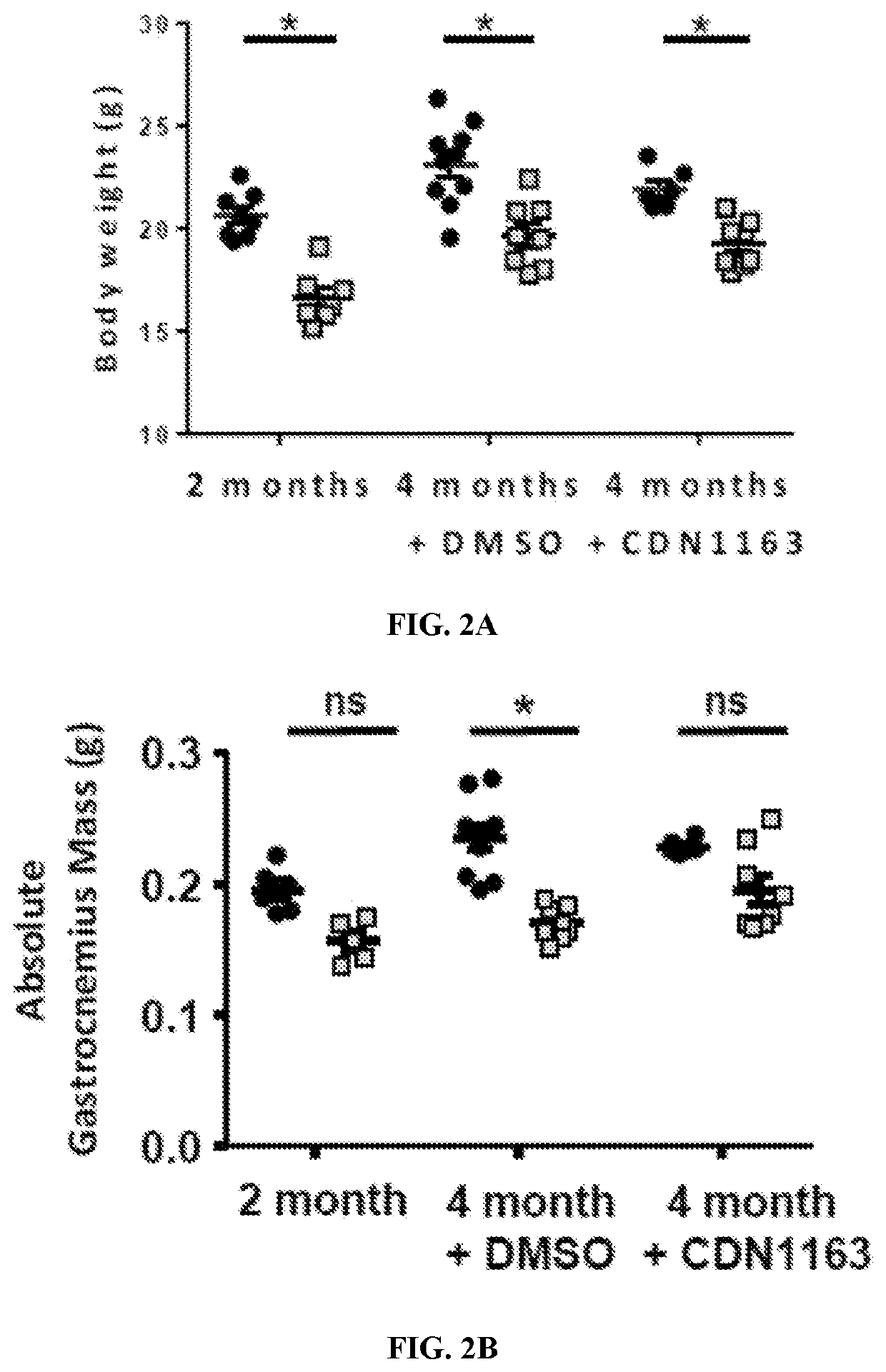
PendingUS20210085676A1Reduced muscle necrosisImproved mitochondrial morphologyCompound screeningApoptosis detectionATPaseSkeletal muscle atrophy
The present invention includes methods and compositions for treating a skeletal muscular atrophy caused by a defect in the function of one or more sarco / endoplasmic reticulum Ca2+-ATPase (SERCA) pumps comprising: identifying a subject having a muscular atrophy caused by a defect in the function of the one or more SERCA pumps, and providing the subject with an effective amount of an activator that enhances an activity of the one or more SERCA pumps.
Owner:OKLAHOMA MEDICAL RES FOUND +1
Portable sitting and standing function evaluation and training device
InactiveCN103190924BReduce power consumptionReduce volumeMuscle exercising devicesDiseaseEngineering
The invention discloses a portable sitting and standing function evaluation and training device which is connected with an upper computer through wireless communication and mainly comprises a pedal module and a cushion module, wherein a power module, a CPU (central processing unit), a foot sole pressure sensor and a Bluetooth module are arranged in the pedal module; a hip pressure sensor is arranged in the cushion module; the foot sole pressure sensor is used for collecting pressure signals of left and right feet; the hip pressure sensor is used for collecting pressure signals of left and right hips; and the CPU is used for processing the pressure signals of the hips and the foot soles and communicates with the upper computer through the Bluetooth module. The sitting and standing functions of a patient are evaluated and trained through sitting and standing function evaluation and training software arranged in the upper computer. The portable sitting and standing function evaluation and training device has the advantages of low power consumption, small volume, convenience for carrying and the like, achieves wireless communication by the Bluetooth module without signal connection wires or cables, is convenient to operate, and can be widely applied to biomedical instruments for treating diseases such as skeletal muscle atrophy, nerve injury, paralysis and the like.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
A pharmaceutical composition of s1pr modulators
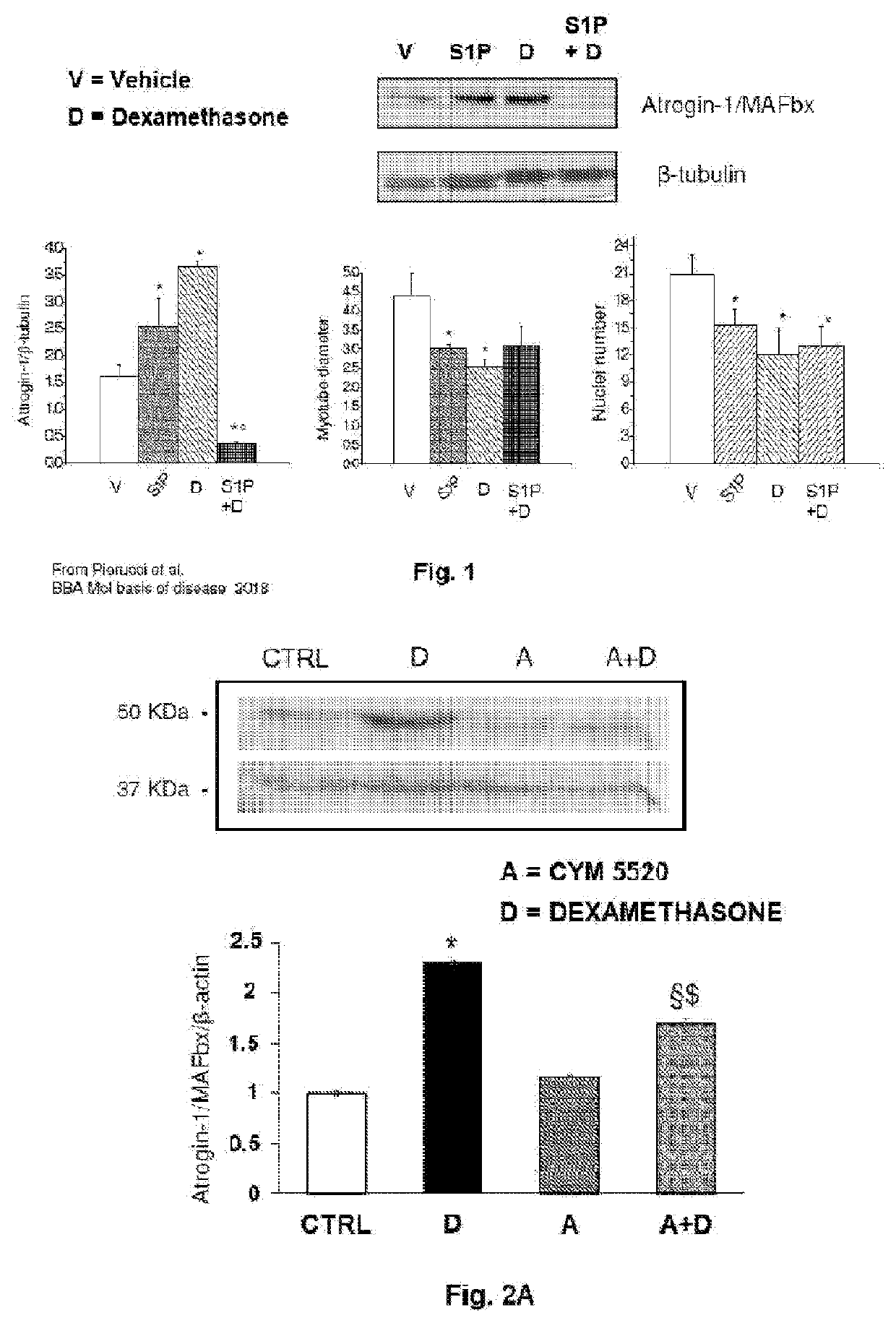
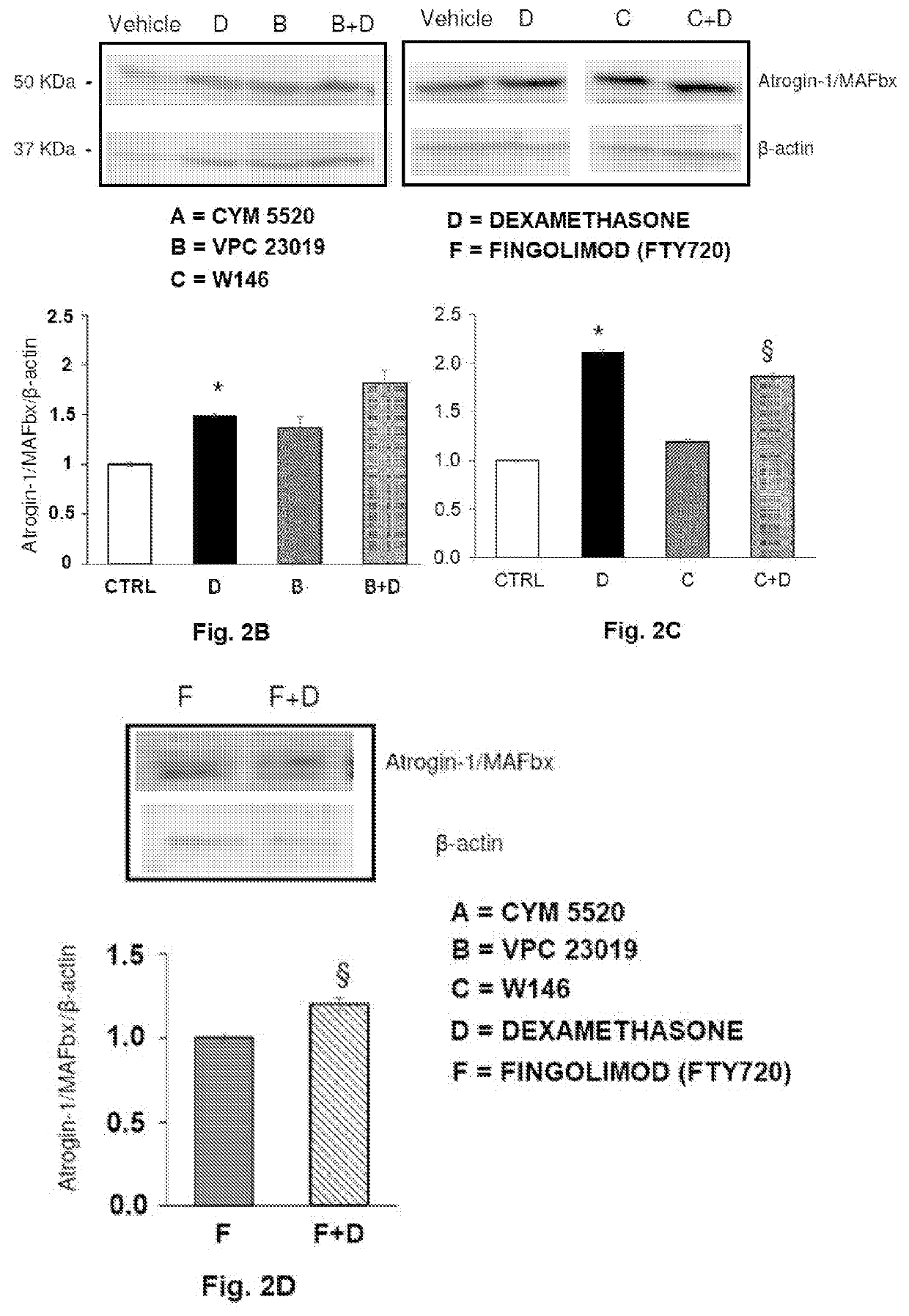
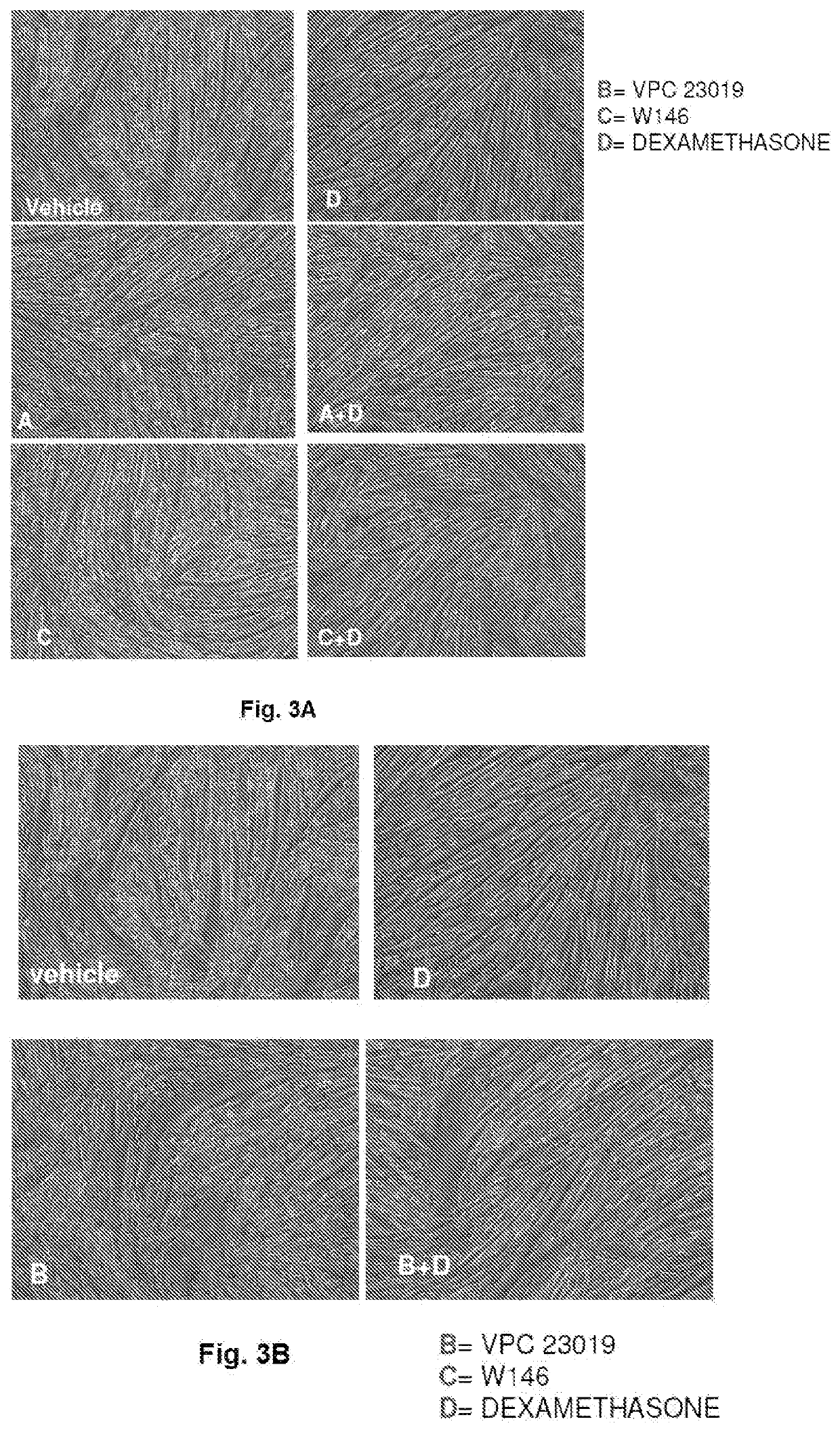
The present invention relates to a pharmaceutical composition useful in the prevention and treatment of atrophy or of degeneration of skeletal muscle caused by pathologies or of sarcopenia. The composition includes a combination of molecules that are modulators of the sphingosine-1-phosphate receptors S1PR and one or more pharmaceutically acceptable excipients and / or carriers.
Owner:UNIVERSITY OF FLORENCE
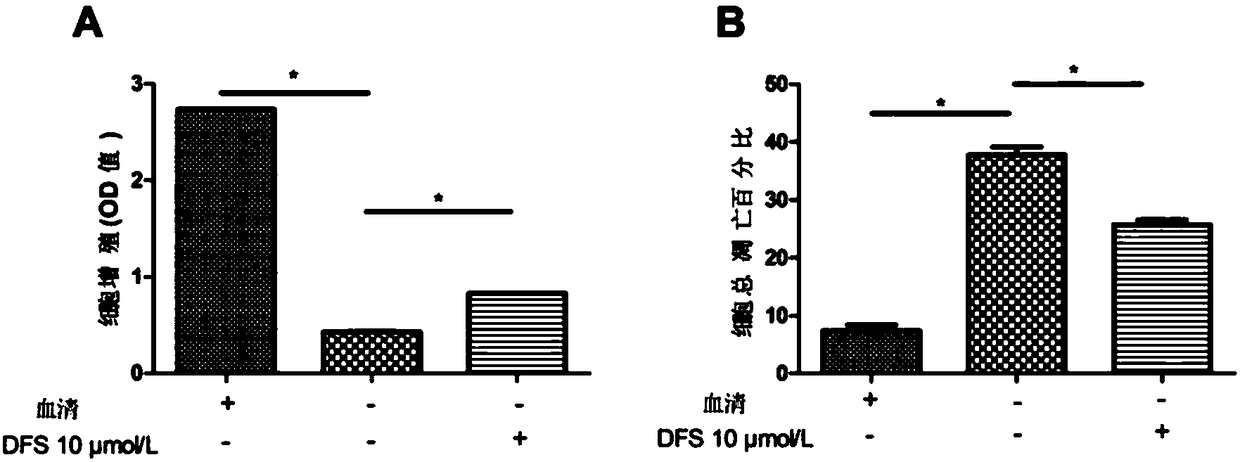
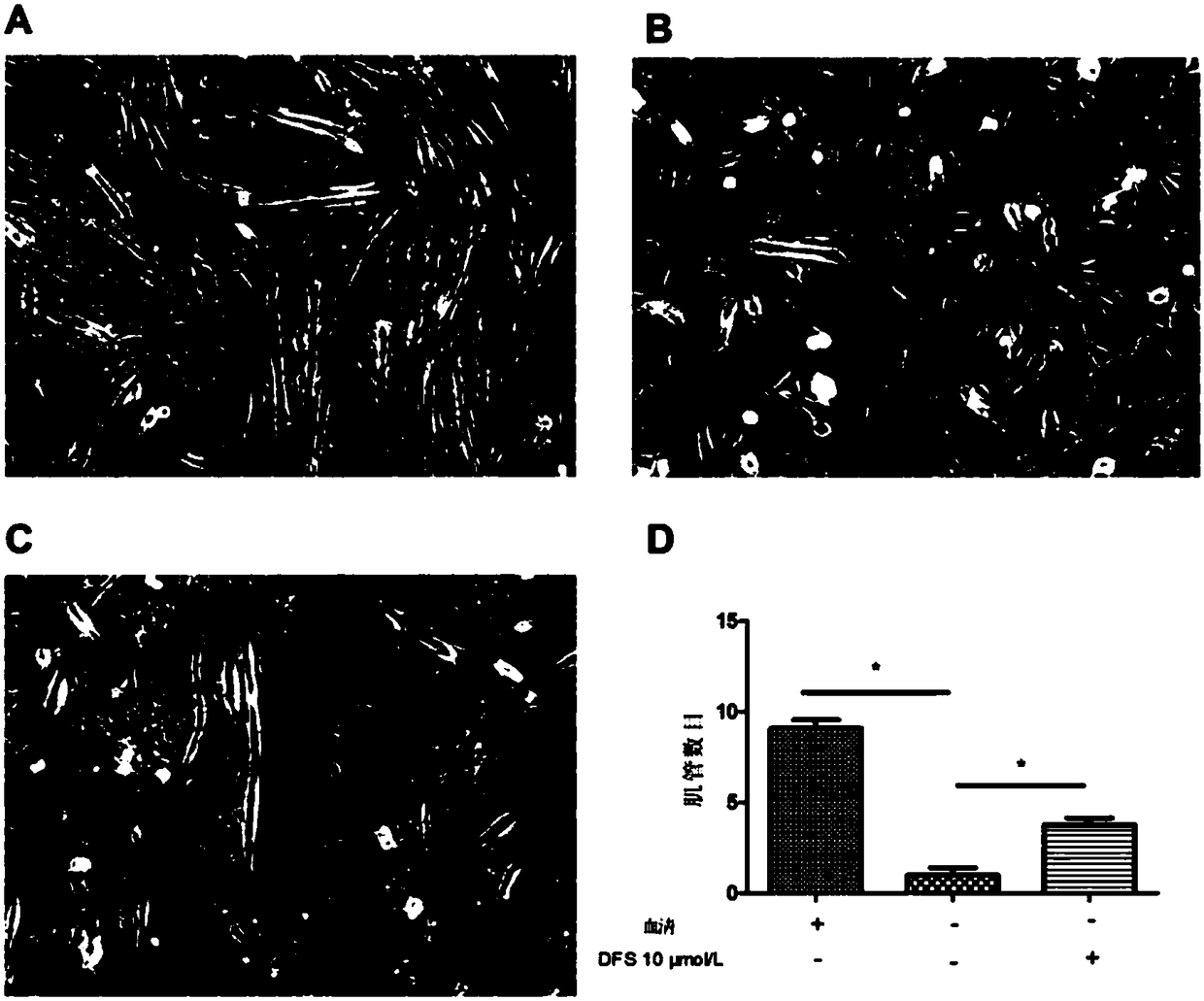
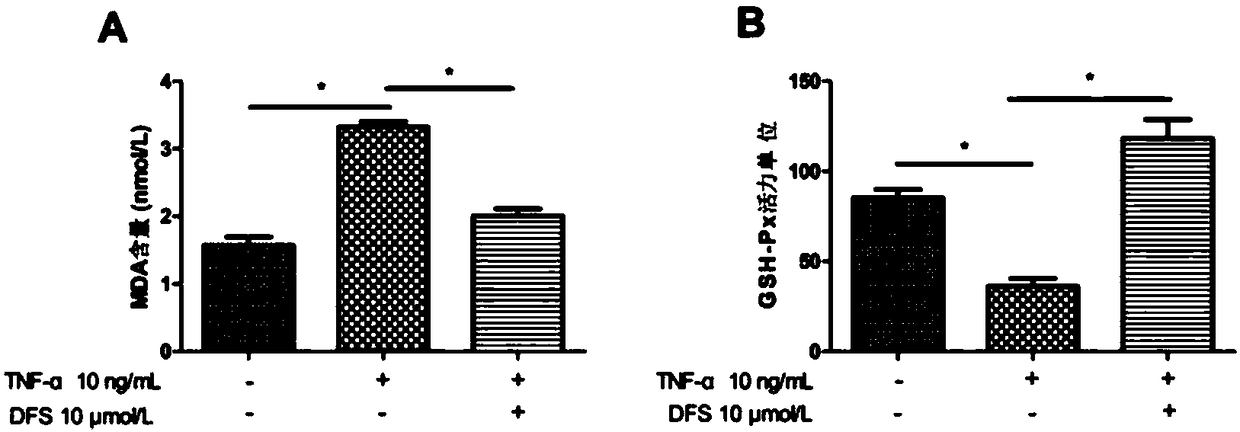
Application of deferasirox to preparation of drug for treating sarcopenia disease
InactiveCN108524501AShort half-lifeImprove complianceOrganic active ingredientsMuscular disorderSkeletal muscle atrophyOxidative stress
The invention relates to novel application of sarcopenia, in particular to application of deferasirox to preparation of a drug for treating the sarcopenia disease, and belongs to the technical field of medical novel application. At present, drugs for treating sarcopenia as a result of an iron accumulation factor are absent at the present clinically. Deferasirox is proved to be capable of remarkably inhibiting skeletal muscle atrophy and oxidative stress injury under a sarcopenia pathology state, and is suitable for developing a prevention drug for the sarcopenia.
Owner:AFFILIATED HOSPITAL OF JIANGSU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com