Medical application of enzymatic hydrolysate of icariin and main component baohuoside I of icariin
A technology of icariin and enzymatic hydrolysis product, which is applied in the fields of medicine and traditional Chinese medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
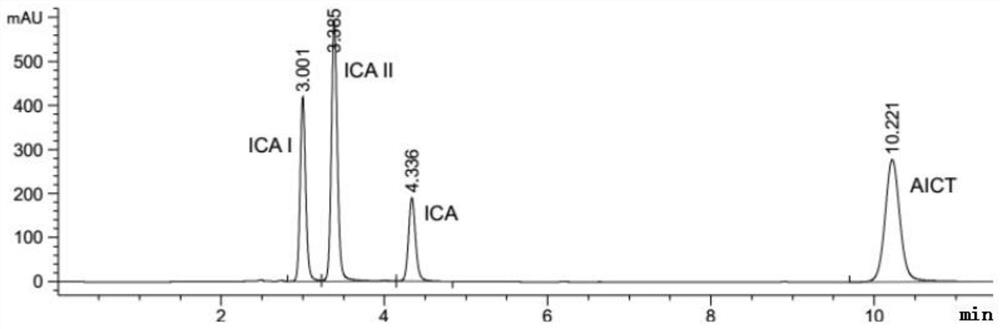
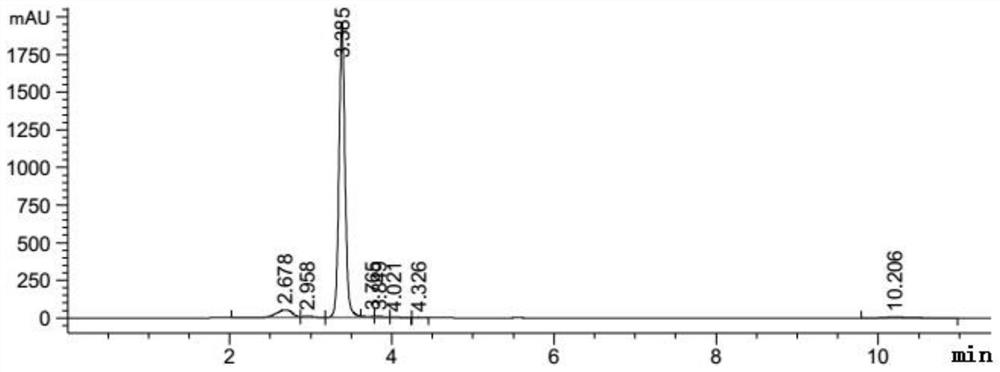
[0031] A preparation method of an enzymatic hydrolysis product of icariin (the main component is baojoside I), the specific steps of which are as follows:
[0032] Weigh about 50 mg of icariin and β-glucosidase, dissolve in 10 mL of 0.2M acetic acid buffer solution with pH=4.0 and stir at 40°C for 8 hours, extract the reaction solution with 50 mL of ethyl acetate for 3 times, collect the upper layer solution, and use Dried over sodium sulfate, concentrated by rotary evaporation, transferred the concentrated solution to a refrigerator at 4°C for static crystallization; dried the crystals in a vacuum at 40°C for 3 hours to obtain the crude product of baochoside I; recrystallized from methanol to obtain the refined product of baochoside I The crude and refined products of glycoside I are the enzymatic hydrolysis products of icariin).
[0033] Adopt HPLC to detect the content of baochoside I in the enzymatic hydrolysis product of icariin (crude product and refined product of baoch...
Embodiment 2
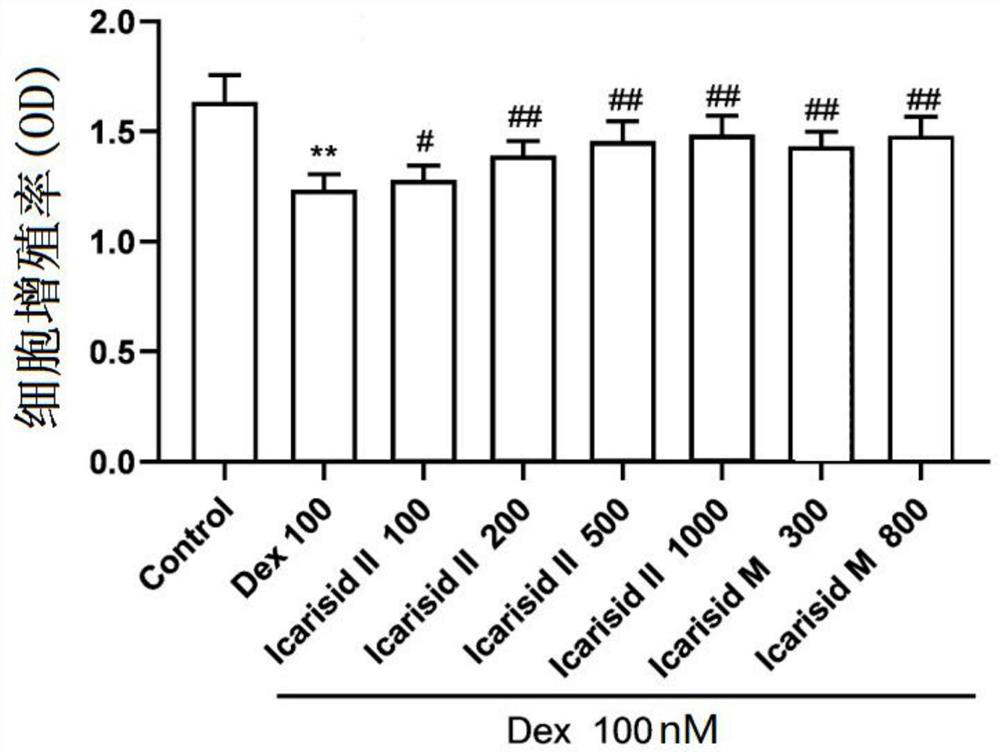
[0035] Test the protective effect of icariin hydrolyzate and baohuoside I on C2C12 myotube cell atrophy induced by dexamethasone:
[0036] The preventive effect of baohuoside I on the myotube cell atrophy model induced by dexamethasone by pre-administration was tested.
[0037] (1) Test method
[0038] A. C2C12 cells were treated with 100 μM Dex (dexamethasone) for 24 hours to establish a model, which was used as a model group (dexamethasone group). Separately set different concentrations of traditional Chinese medicine monomer groups, the specific method is: add different concentrations of baohuoside I (Icarisid II) 12 hours in advance of adding dexamethasone, so that the final concentration is 100, 200, 500, 1000nM, and different concentrations The final concentration of icariin hydrolyzate (Icarisid M) was 300, 800 nM.
[0039] Results: Compared with the dexamethasone group, each concentration of Icarisid II and Icarisid M could promote cell proliferation, and the concent...
Embodiment 3
[0057] Test the protective effect of icariin hydrolyzate and baohuoside I on dexamethasone-induced skeletal muscle atrophy in mice:
[0058] The enzymatic hydrolysis product of icariin and baohuoside I were tested for the prevention of dexamethasone-induced skeletal muscle atrophy in mice by intragastric administration.
[0059] (1) Test method
[0060] Normal group (abbreviated as Normal): intraperitoneal injection of normal saline, and gavage of 50 μl of 0.1% ethanol at the same time, for 14 consecutive days, once a day. Model group (Dex for short): intraperitoneal injection of dexamethasone solution, 1 mg / kg.day, and 50 μl of 0.1% ethanol, for 14 consecutive days, once a day. Baohuoside I group (referred to as Dex+Icarisid II) intraperitoneally injected dexamethasone solution, 1mg / kg.day, and at the same time 0.005mg / 10g body weight 0.005mg / 10g Baohuoside I solution 50 microliters into the stomach, continuous perfusion for 14 days, 1 times / day. Icariin hydrolyzate group ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com