Sesquiterpene lactone compounds as well as preparation method and application thereof
A technology of ester compounds and sesquiterpenes, applied in the field of sesquiterpene lactone compounds, can solve the problems of body discomfort, shorten the lifespan of patients, worsen malignant tumors, etc., so as to slow down weight loss, reduce tumor load, inhibit protein Degradation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The molecular structure of the sesquiterpene lactone compound (referred to as PZ-1 in this example) in this example is as follows:
[0049]
[0050] The synthetic method of sesquiterpene lactone compound described in the present embodiment is as follows:
[0051] After drying 10kg of the leaves, reflux extraction with 95% ethanol (with 5% water) was carried out twice, each time for 2 hours, and the extracts were combined. Concentrate to remove ethanol.
[0052] The concentrate was extracted with petroleum ether, and the petroleum ether extract was discarded; the remaining concentrate was extracted with ethyl acetate, and the ethyl acetate extracts were combined, and the ethyl acetate was removed to obtain chrysanthemum lactone PZ-1. Further, it was purified by column chromatography (eluted with a mixed solvent of chloroform / methanol).
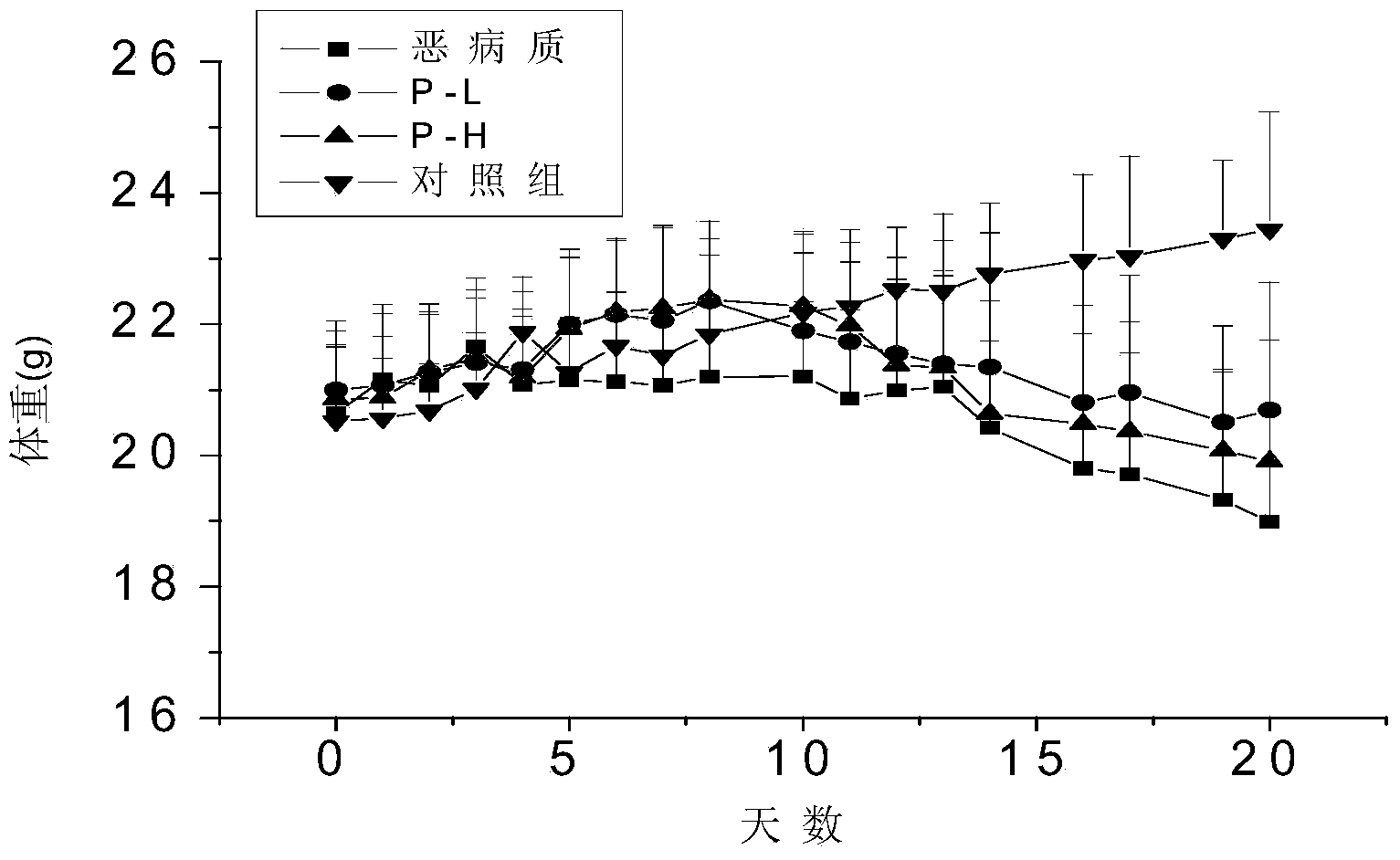
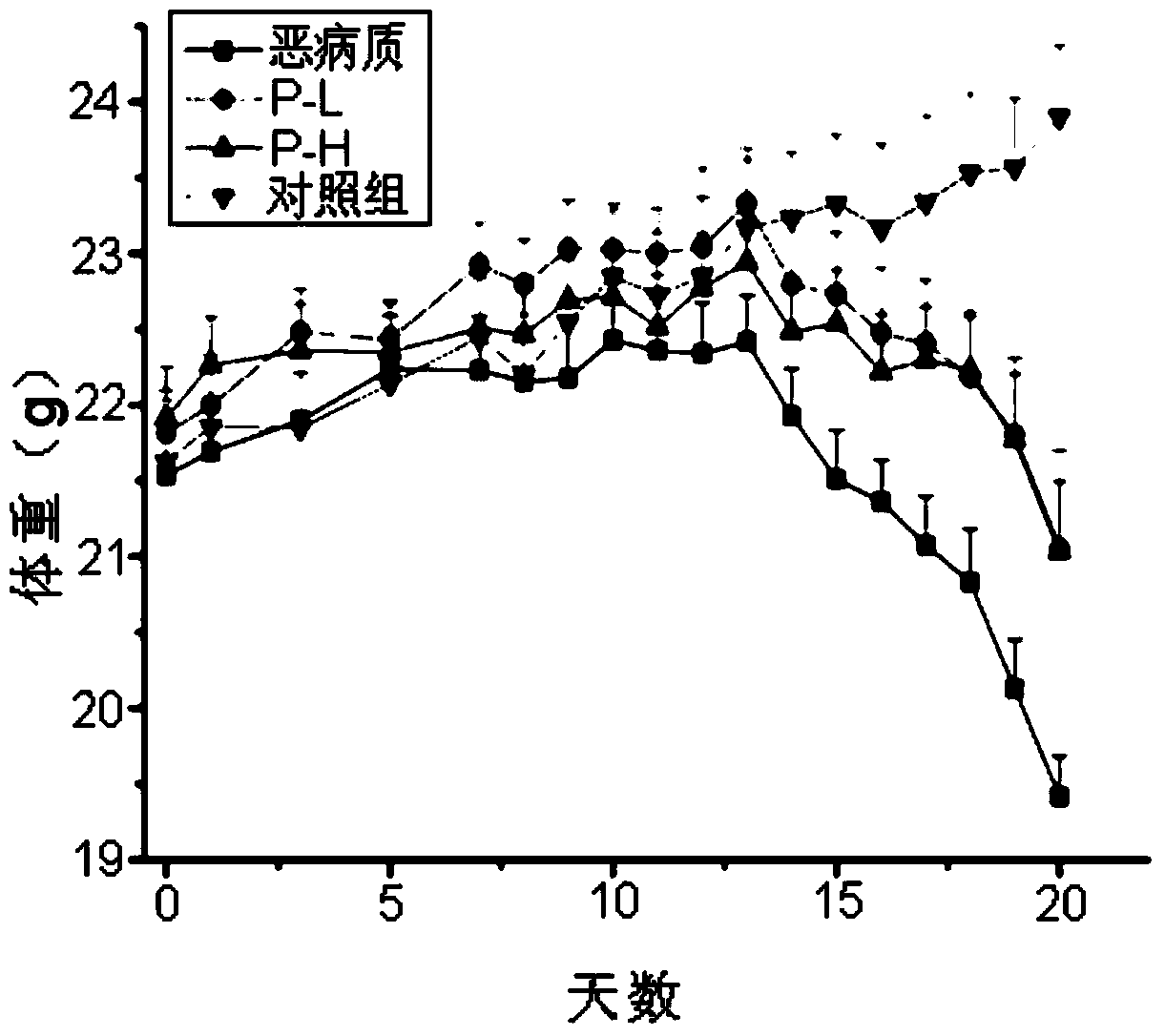
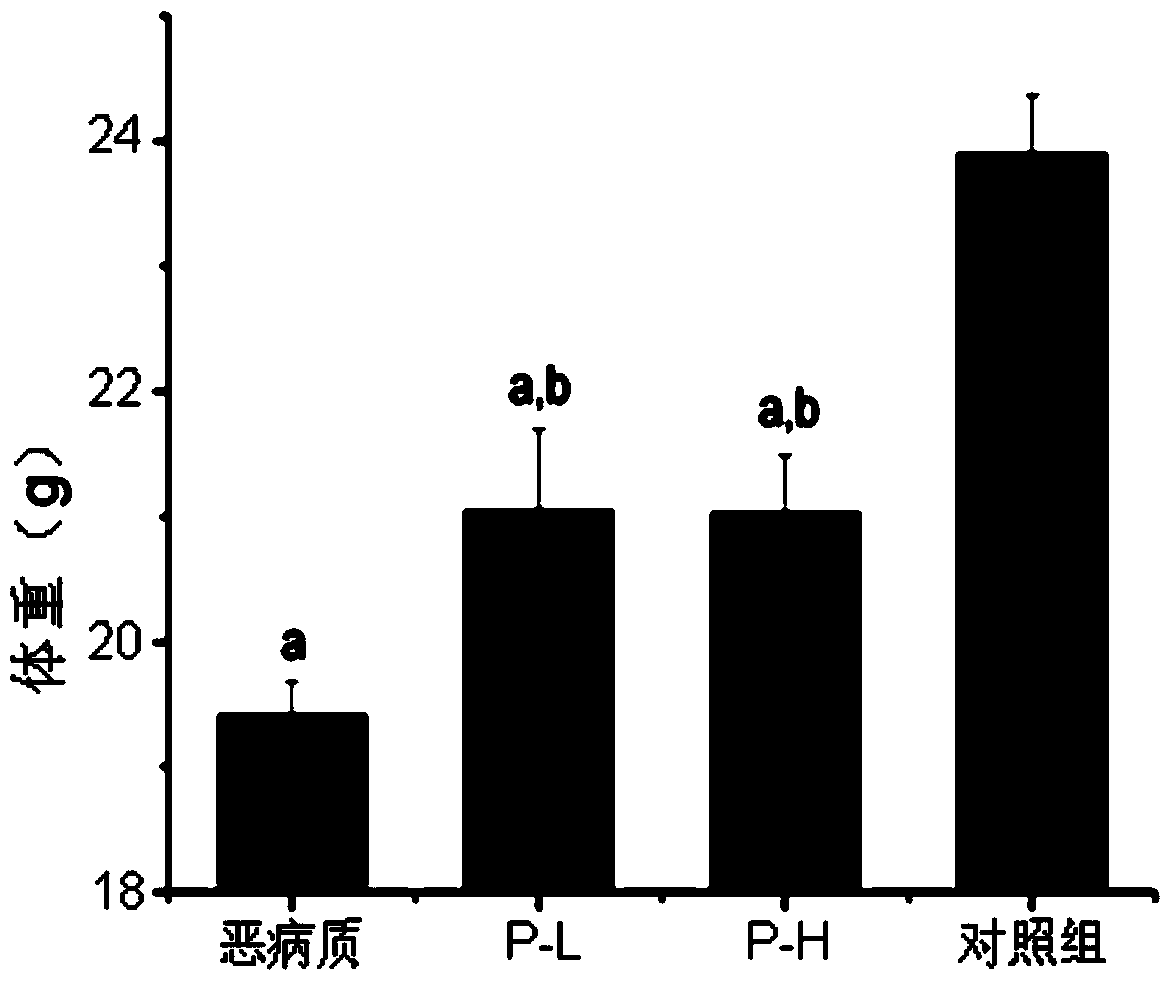
[0053] Prevention of weight loss in cachexia mice
[0054] Material: PZ-1 prepared by separation and purification in this e...
Embodiment 2
[0071] The molecular structure of the sesquiterpene lactone compound (referred to as PZ-2 in this example) in this example is as follows:
[0072]
[0073] Wherein, Bn is benzyl.
[0074] The synthetic method of sesquiterpene lactone compound described in the present embodiment is as follows:
[0075] R in PZ-1 3 (Methyl) halogenation followed by reaction with BnONa afforded PZ-2. The reaction formula is as follows:
[0076]
Embodiment 3
[0078] The molecular structure of the sesquiterpene lactone compound (referred to as PZ-3 in this example) in this example is as follows:
[0079]
[0080] The synthetic method of sesquiterpene lactone compound described in the present embodiment is as follows:
[0081]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com