Alk5 inhibitors as skeletal muscle hypertrophy inducers
An inhibitor, skeletal muscle technology, applied in the field of ALK5 inhibitor of skeletal muscle hypertrophy inducer, can solve the problem that there is no specific treatment to stop or reverse muscular dystrophy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0185] Materials and methods
[0186] Cell Sources and Cell Culture
[0187] Healthy donor primary skeletal cells (Donor 1, Donor 3, and Donor 5) were Clonetics TM Human skeletal muscle myoblasts (HSMM). In addition, cells were derived from three DMD (Duchenne muscular dystrophy) donors (donors Z, W and D1) and a healthy donor (donor A).
[0188] Donor characteristics are detailed in Table 1 below.
[0189] Table 1: Donor Characteristics
[0190]
[0191]
[0192] Muscle cell cultures were maintained with supplements and fetal bovine serum (FBS) serum provided by Lonza following the supplier's instructions. An expansion step is performed to obtain enough cells to seed selection plates.
[0193] Hypertrophy Assay and Atrophy Rescue Assay
[0194] Using a file called MyoScreen TM Hypertrophy assays and atrophy rescue assays were performed on an in vitro fully automated human myotube model (Cytoo, France). This model relies on tight control of the microenviron...
Embodiment 2
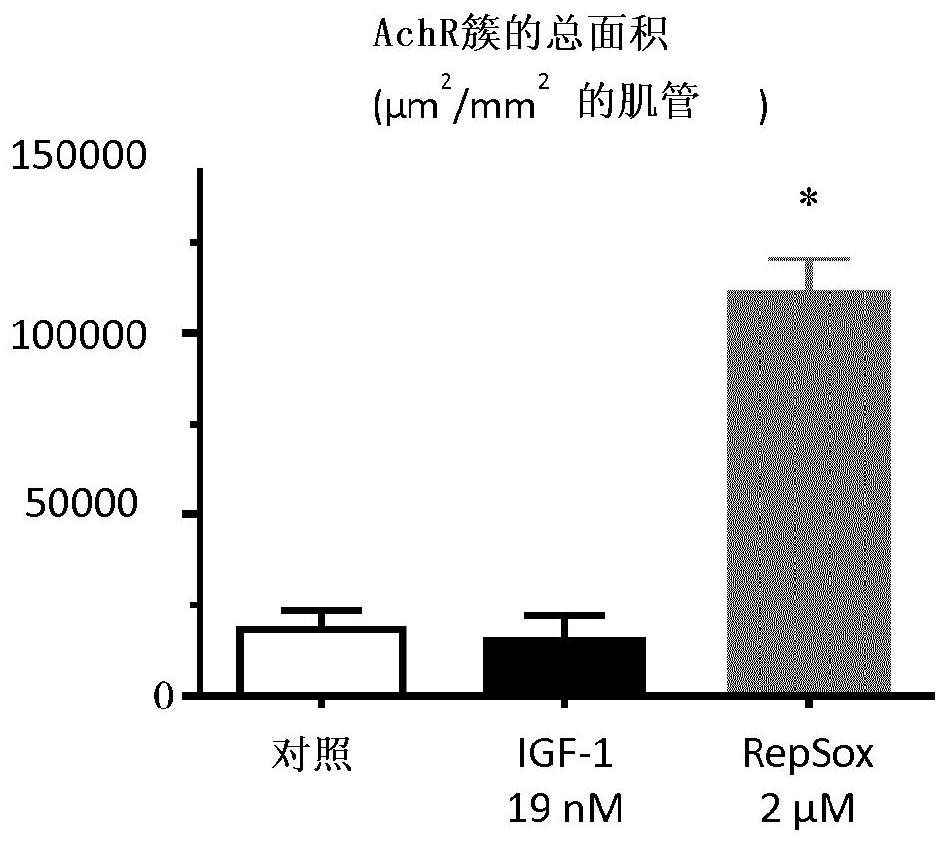
[0236] Example 2: Acetylcholine Receptor Clustering Assay
[0237] Materials and methods
[0238] Perform MyoScreen as described in the hypertrophy and atrophy rescue assay described in Example 1 TMregimen, but discontinued on day 9 instead of day 6. At the end of the assay, AchR was immunostained using specific antibodies in addition to troponin T and nuclei. Images were acquired at ×20 using a Perkin Elmer Operetta high-content imaging system. Image processing and analysis were performed using dedicated algorithms developed on Acapella high-content imaging software (Perkin Elmer). Specific readouts were calculated for each well: nuclei count and myotube fusion index, AchR number, AchR average area, total AchR area normalized by total myotube area.
[0239] result
[0240] Notably, when labeled with a specific anti-AChR antibody, MyoScreen myotubes 6 days after differentiation showed that AChR clusters were interrupted from time to time along the sarcolemma at differ...
Embodiment 3
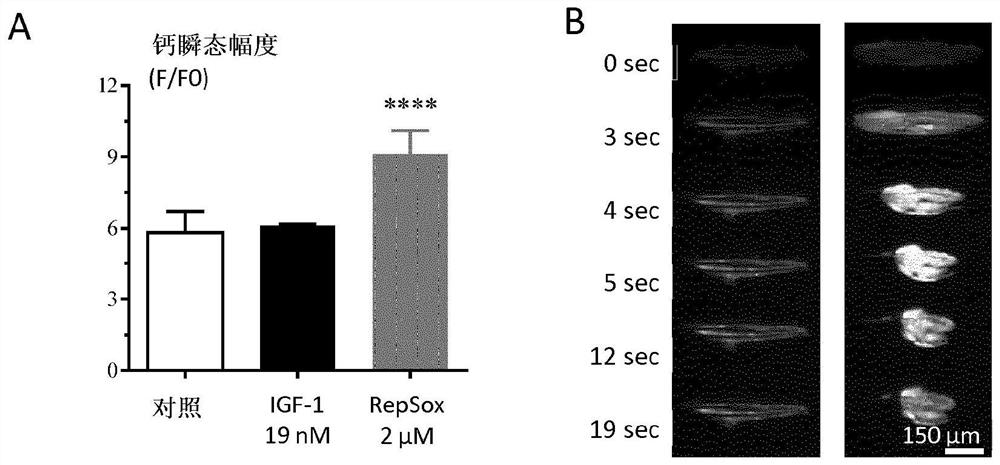
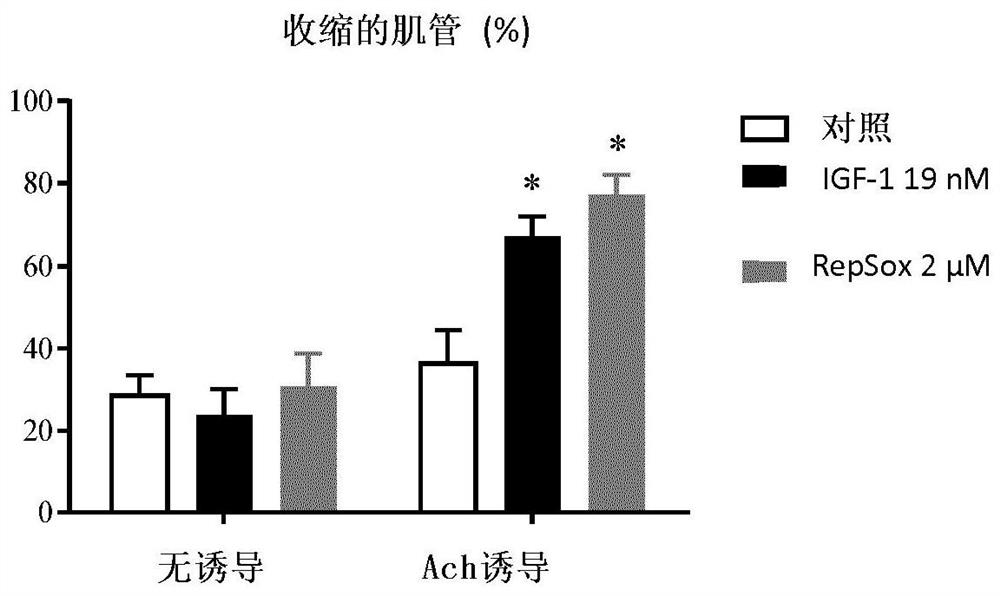
[0241] Example 3: Calcium flux assay
[0242] Materials and methods
[0243] The cells were washed with a calcium buffer containing (in mM): 130 NaCl, 5.4 KCl, 1.8 CaCl2, 0.8 MgCl2, 5.6 D-glucose, 10 Hepes, pH 7.4. Cells were then incubated with 2 μM Fluo-4 AM (F14201-Invitrogen) for 1 hour in an incubator (37° C., 5% CO 2 ). Myotube responses to 20 μM ACh (A6625-Sigma-Aldrich) will be video imaged after washing to remove unloaded dye. The interval of Streamacquisitions was set to 1 second, and one field of view was collected per well. Images were processed and analyzed automatically using proprietary software developed in-house that incorporates the open source ImageJ framework. This image analysis tool segments myotubes within images acquired in the same well. The total Fluo-4 fluorescence intensity inside each myotube was measured and corrected for background. Total intensity was normalized to myotube area to obtain mean fluorescence intensity readings. For data pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com