Fentanyl composition for treatment of acute pain
An acute and combination technology, applied in the direction of active ingredients of heterocyclic compounds, non-central analgesics, drug combinations, etc., can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The invention will be described in more detail below with reference to examples, which give preferred but non-limiting embodiments. Example 1. Preparation of rapidly disintegrating tablets with bio / mucoadhesion promoting properties
[0055] Tablet batches of 1000 were prepared with the following composition: 81.5 g of mannitol and 2.0 g of Ac-Di-Sol(R) (disintegrant and bio / mucoadhesion promoter) were mixed with about 170 ml of absolute ethanol. The dried mixture was pressed through a metal sieve with a pore size of 1 mm and the resulting fraction having a particle size of about 250 to 450 microns was mixed with 500 mg of micronized fentanyl and 1.0 g of finely ground sodium lauryl sulfate (surfactant) 50 hours. The resulting mixture was mixed with 5.0 g of Avicel(R) Ph 101 and 10.0 g of sodium alginate (bio / mucoadhesion promoter and disintegrant) for 60 minutes. The resulting mixture was compressed under a pressure of 200 MPa into tablets, each weighing 100 mg and co...
Embodiment 2
[0056] The dissolution rate of the prepared tablets was determined according to USP XXIII (paddle method) at two different stirring speeds (25 and 100 rpm). Example 2. Preparation of rapidly disintegrating tablets with bio / mucoadhesion promoting properties
[0057] Tablet batches of 1000 were prepared with the following composition: 91.0 g of mannitol (granules with a particle size of 250-450 μm) were mixed with 1.0 g of sodium lauryl sulfate and 500 mg of micronized fentanyl in a V-blender 24 hours. It was then mixed with 5.0 g of Avicel(R) Ph101 and 2.0 g of Ac-Di-Sol(R) (used both as a disintegrant and as a bio / mucoadhesion promoter) for 2 hours. Finally, it is mixed with 0.5 g of magnesium stearate for 2 minutes. The formed tablet pellets were compressed into tablets under a pressure of 130 MPa, each containing 0.5 mg of fentanyl.
[0058] The disintegration time was tested using the apparatus described in Ph. Eur. (latest edition).
[0059] The measured disintegration...
Embodiment 3
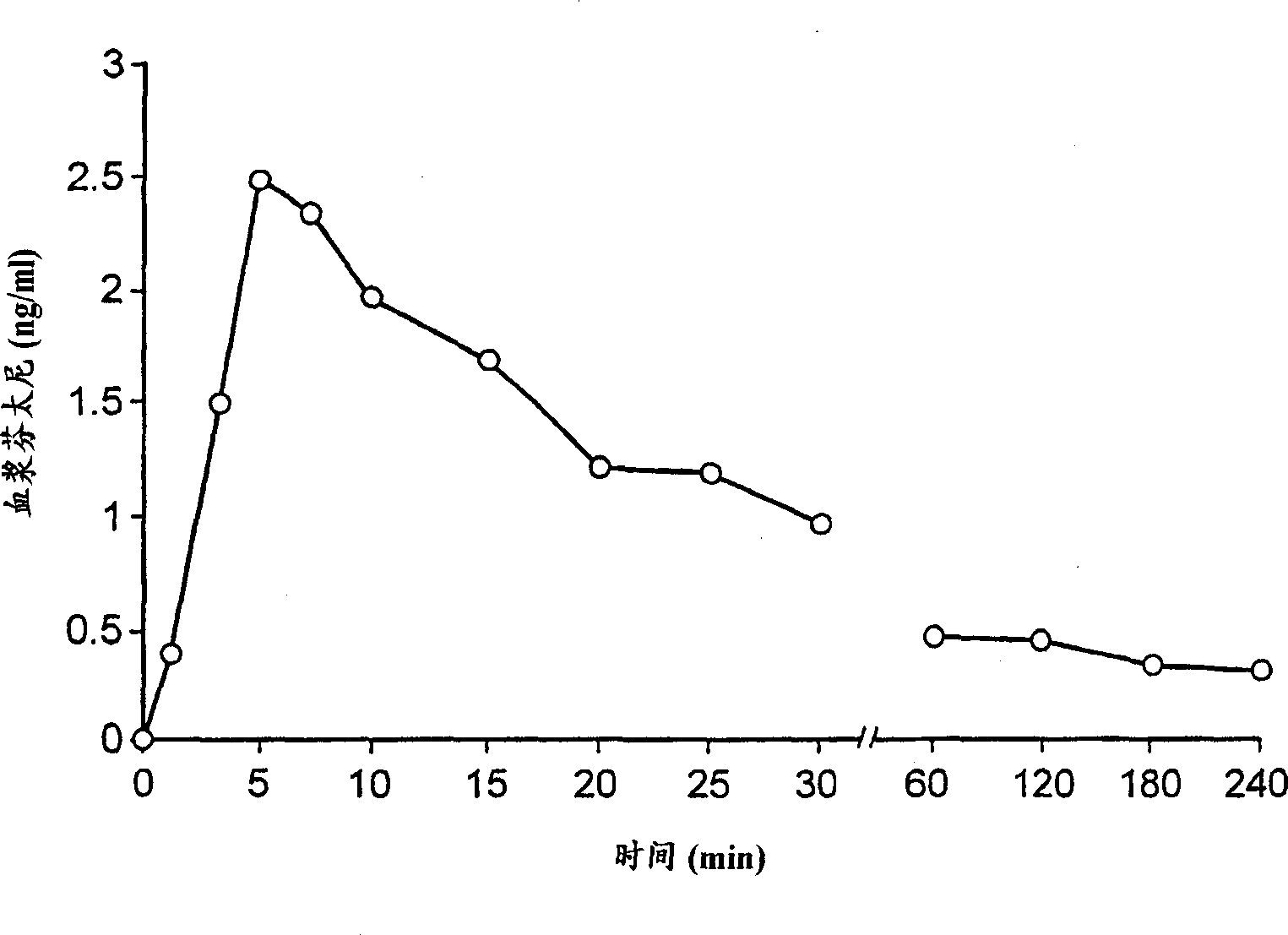
[0062]A patient suffering from breakthrough pain due to cancer was administered 400 μg of fentanyl in the form of a sublingual tablet formulated as described in Example 1 . Plasma concentrations of fentanyl were monitored for 240 minutes after dosing and the results are shown in the accompanying drawings. It can be seen that the uptake of fentanyl is very rapid, reaching a maximum already after 5 minutes. This indicates that the sublingual formulation of the present invention allows rapid ingestion of the active ingredient, although only a small amount of liquid is available to dissolve the active ingredient in this route of administration. Example 4. Evaluation of biological / mucoadhesive properties
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com