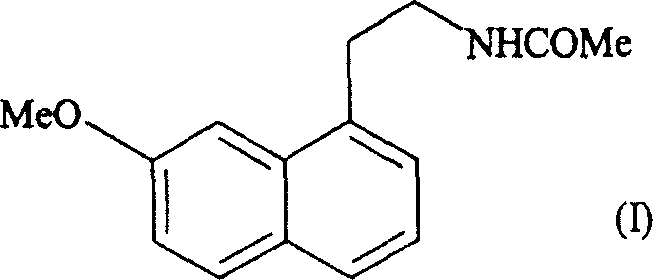
Orodispersible pharmaceutical composition for oromucosal or sublingual administration of agomelatine
A composition and drug technology, applied in the directions of drug combination, drug delivery, sugar-coated pills, etc., can solve the problems of unresearched pharmacological properties, etc., and achieve the effect of good acceptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Formulation: 320mg prepared tablet
[0055] components
Embodiment 2
[0057] Formulation: 350mg prepared tablet
[0058] components
Quantity (mg)
central nucleus
Starlac
Powdered Anhydrous Citric Acid
Starlac
1
48.75
0.25
10
282
3
3
2
[0059] The central core was made by mixing the components followed by direct compression. The cores of Examples 1 and 2 had a hardness of about 15 Newtons and a brittleness of less than 1%. The components of the coating layer are mixed and the coating is carried out by compression of the obtained powder mixture around the core.
[0060] The coated tablets of Examples 1 and 2 had a target hardness of 40 Newtons and a friability of about 1%. The in vitro disintegration time is less than 3 minutes (European Pharmacopoeia).
Embodiment 3
[0062] Formulation: 320mg prepared tablet
[0063] components
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com