Composition containing xanthophylls/lutein ester and application thereof
A technology of lutein esters and lutein, applied in the field of lutein/lutein ester preparations, can solve the problem of reducing the bioavailability of lutein/lutein esters and the stability of lutein/lutein esters Sexual damage and other problems, achieve significant health care and therapeutic effects, increase eye blood flow, nutrition and health care taste effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
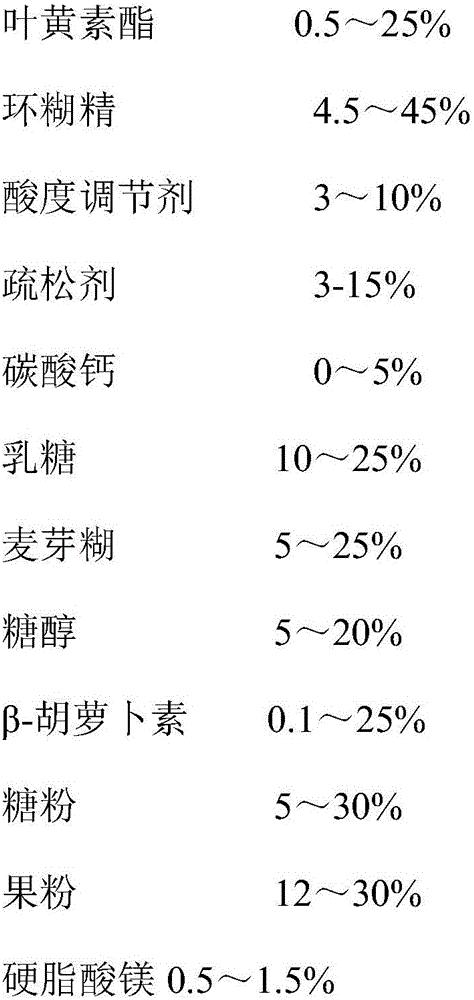
[0028] Recipe: (weight)
[0029]
[0030]
[0031] Preparation:
[0032] The components are mixed and then prepared into orally disintegrating tablets by methods known in the art. Clinical Observation:
[0033] (1) Clinical data
[0034] A clinical trial was carried out on 220 outpatients. The clinical manifestations of these patients were dim eyesight, eye pain, weak dark adaptation, blurred vision, photophobia, dry eyes, and blinking. They were non-hospitalized and voluntarily participated in the experiment; 110 cases in the treatment group ( Male: female = 1:1), and 110 cases in the control group (male: female = 1:1).
[0035] (2) Treatment method
[0036] The control group used Zhenshiming Eye Drops, once a day, and 15 days was a course of treatment; the treatment group took the compound of the present invention orally, 550 mg / tablet, 4 tablets per day;
[0037] (3) The clinical trial results are as follows:
[0038]
[0039] There is a significant differenc...
Embodiment 2
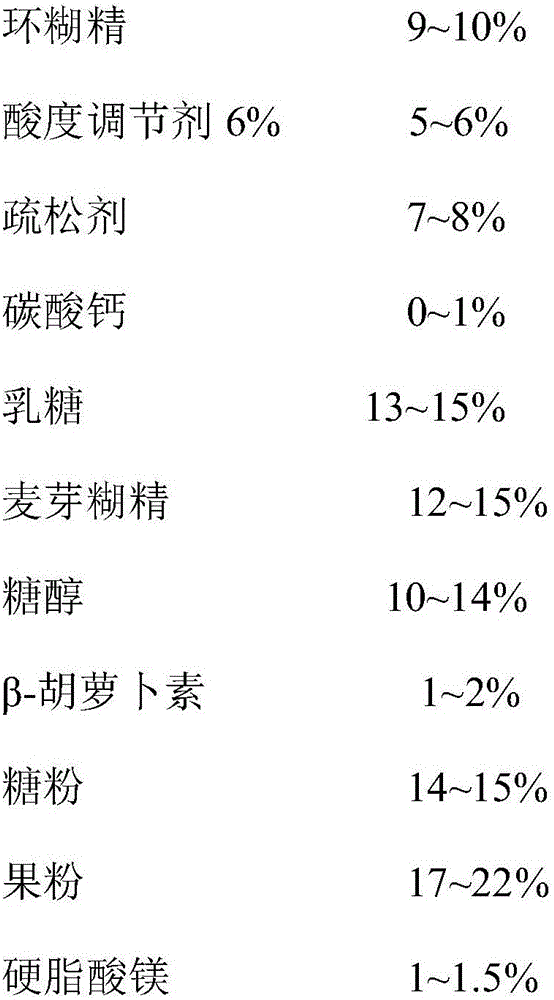
[0041]
[0042] Clinical Observation:
[0043] (1) Clinical data
[0044] A clinical trial was carried out on 180 outpatients. The clinical manifestations of these patients were dim eyesight, eye pain, weak dark adaptation, blurred vision, photophobia, dry eyes, and blinking. They were non-hospitalized and voluntarily participated in the experiment; 100 cases in the treatment group ( Male: female = 1:1), and 80 cases in the control group (male: female = 1:1).
[0045] (2) Treatment method
[0046] The control group uses eye drops, once a day, and 15 days is a course of treatment; the treatment group takes orally the compound of the present invention, 550 mg / tablet, 4 tablets per day;
[0047] (3) The clinical trial results are as follows:
[0048]
[0049]
[0050] There are significant differences between the treatment group and the matched group, and it is concluded that the clinical application of the present invention has a significant curative effect.
[0051...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com