Transdermal medicament delivery system containing donepezil compound, preparation and preparation method
A transdermal drug delivery system, the technology of donepezil, applied in the field of transdermal drug delivery systems, can solve the problems of poor compliance and high clinical dropout rate of AD patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
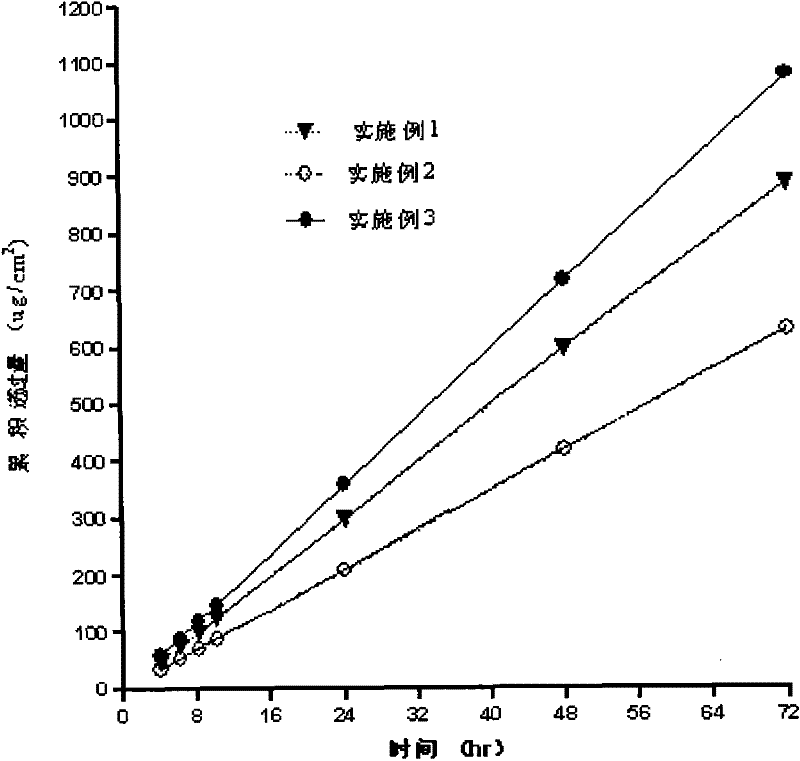
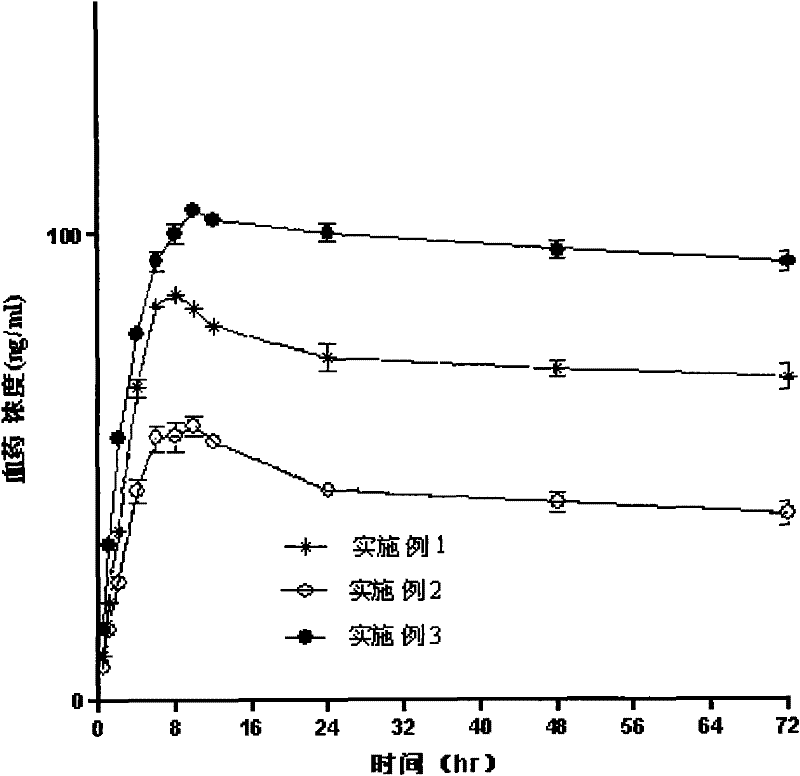
Embodiment 1
[0102] [Prescription] (based on 2000 tablets)
[0103] Donepezil 55g, hydrophobic skeleton matrix acrylic pressure-sensitive adhesive 895.5g, humectant propylene glycol 20g, transdermal penetration enhancer isopropyl myristate 20g, pH regulator triethanolamine 9g, bacteriostatic agent methylparaben 0.5g; Total: 1000g
[0104] Preparation:
[0105] Add donepezil and methylparaben to a mixed solution of 400 g of glacial acetic acid and dichloromethane (glacial acetic acid:dichloromethane=1:2, volume ratio) to dissolve, and stir at 5000 rpm for 0.5 hours to obtain a medicinal solution;
[0106] Add acrylic pressure-sensitive adhesive and isopropyl myristate to the above drug solution, mix well, then add propylene glycol, and stir at a speed of 10,000 rpm for 1 hour, mix and disperse to obtain drug-containing glue.
[0107] Finally, add triethanolamine to the drug-containing colloid, mix, disperse, and degas to obtain the drug-containing colloid.
[0108] Coat the drug-containi...
Embodiment 2
[0112] [Prescription] (based on 2000 tablets)
[0113] Donepezil hydrochloride 80g, hydrophilic polymer matrix sodium polyacrylate 60g, transdermal penetration enhancer azone 96g, humectant glycerin 150g, crosslinking agent aluminum oxide 5g, acidic pH regulator tartaric acid 2g, alkaline pH regulator III Ethanolamine 54g, antibacterial agent benzyl alcohol 3g; solvent water 550g; total: 1000g
[0114] Preparation method: add donepezil hydrochloride, benzyl alcohol and tartaric acid to water and dissolve to obtain medicinal solution A; put sodium polyacrylate and aluminum oxide together in glycerin to suspend and swell for 2 hours to obtain sodium polyacrylate suspension B ; Slowly add the medicinal solution A to the above-mentioned sodium polyacrylate suspension B, while stirring at a speed of 10000 rpm for 1 hour, mix and disperse to obtain a hydrogel solution. Then add azone to the above-mentioned hydrogel liquid, mix and disperse, finally add triethanolamine, adjust the p...
Embodiment 3
[0119] [Prescription] (based on 2000 tablets)
[0120] Donepezil hydrochloride 100g, solvent hydroalcoholic solution (water:ethanol=6:4, volume ratio) 200g, polyvinylpyrrolidone (K-30) 70g, acrylic pressure-sensitive adhesive 400g, lauryl lactate 150g, propylene glycol 10g, pH Regulator sodium hydroxide 60g, benzalkonium chloride 10g, total: 1000g.
[0121] Preparation:
[0122] Add donepezil hydrochloride, benzalkonium chloride and polyvinylpyrrolidone (K-30) together to 150g ethanol solution (water: ethanol = 6: 4, volume ratio) after swelling for 4 hours, then add sodium hydroxide solution (hydrogen Add sodium oxide to 50 g of ethanol solution (water: ethanol = 6: 4, volume ratio) to dissolve), mix, adjust the pH to 7.5, and obtain drug reservoir A;
[0123] Add acrylic pressure-sensitive adhesive, propylene glycol and lauryl lactate to the above drug reservoir A, and stir at a speed of 10,000 rpm for 1 hour, mix and disperse, and obtain viscose B containing the drug rese...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com