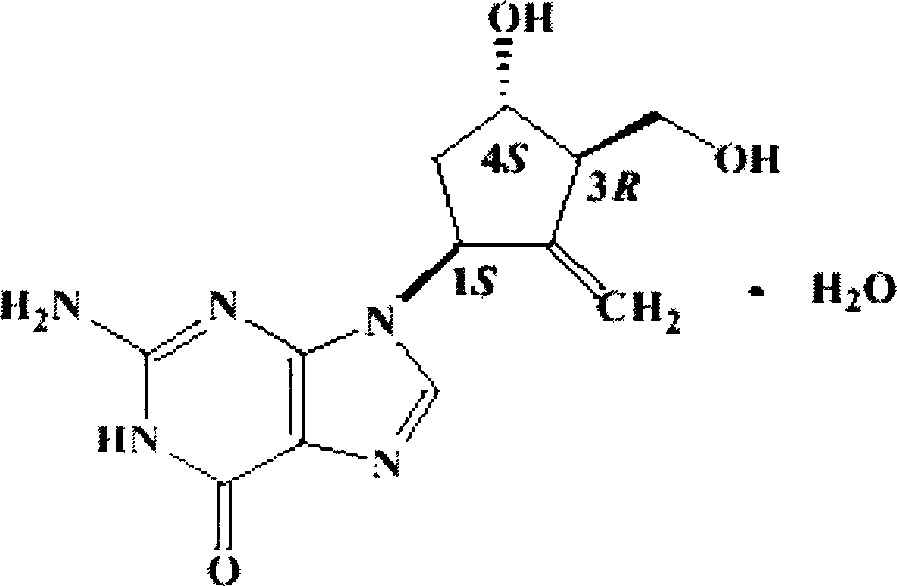
Improved entecavir oral disintegrating tablet and its preparing method
A technology of orally disintegrating tablets and entecavir, which is applied in the field of improved entecavir orally disintegrating tablets and its preparation, can solve problems such as single method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] prescription:
[0030] Entecavir 0.5g
[0031] Crospovidone 60g
[0032] Microcrystalline Cellulose 40g
[0033] Lactose 15g
[0034] Sucrose 15g
[0036] Eudrgit L30D 7.5g
[0037]Orange essence 0.5g
[0038] A total of 1000 pieces were made
[0039] Preparation:
[0040] Add Entecavir to Eudrgit L30D suspension, add appropriate amount of water, stir evenly, then add crospovidone, microcrystalline cellulose, lactose, sucrose, sodium saccharin, orange essence, and stir while adding to form a suspension , pouring into a suitable mold, freeze-drying, pressing and sealing, and packaging.
Embodiment 2
[0042] prescription:
[0043] Entecavir 0.1g
[0044] Mannitol 20g
[0045] 20g pregelatinized starch
[0046] Guar gum 8g
[0047] Gum Arabic 12g
[0048] Xylitol 30g
[0049] Microcrystalline Cellulose 15g
[0050] Lemon essence 1g
[0051] A total of 1000 pieces were made
[0052] Preparation:
[0053] Add entecavir to the mixture of guar gum and gum arabic, stir evenly, then add mannitol, pregelatinized starch, xylitol, microcrystalline cellulose, lemon flavor, stir while adding, after forming a suspension, pour Put in a suitable mold, freeze-dry, press seal, and pack.
Embodiment 3
[0055] prescription:
[0056] Entecavir 0.25g
[0057] Gelatin 25g
[0058] Xylitol 20g
[0059] Microcrystalline Cellulose 40g
[0060] Aspartame 2.5g
[0061] Cross-linked sodium carboxymethyl starch 8.0g
[0062] Peach flavor 0.5g
[0063] A total of 1000 pieces were made
[0064] Preparation:
[0065] Add entecavir into the gelatin liquid, stir evenly, add xylitol, microcrystalline cellulose, aspartame, croscarmellose sodium starch, peach essence, stir while adding, and pour into Put in a suitable mold, freeze-dry, press seal, and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com