External medicine combination for treating skin allergic disease
A technology for external use of drugs and compositions, applied in allergic diseases, skin diseases, drug combinations, etc., can solve the problems of inevitable adverse drug reactions and unsatisfactory effects, and achieve a good synergistic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
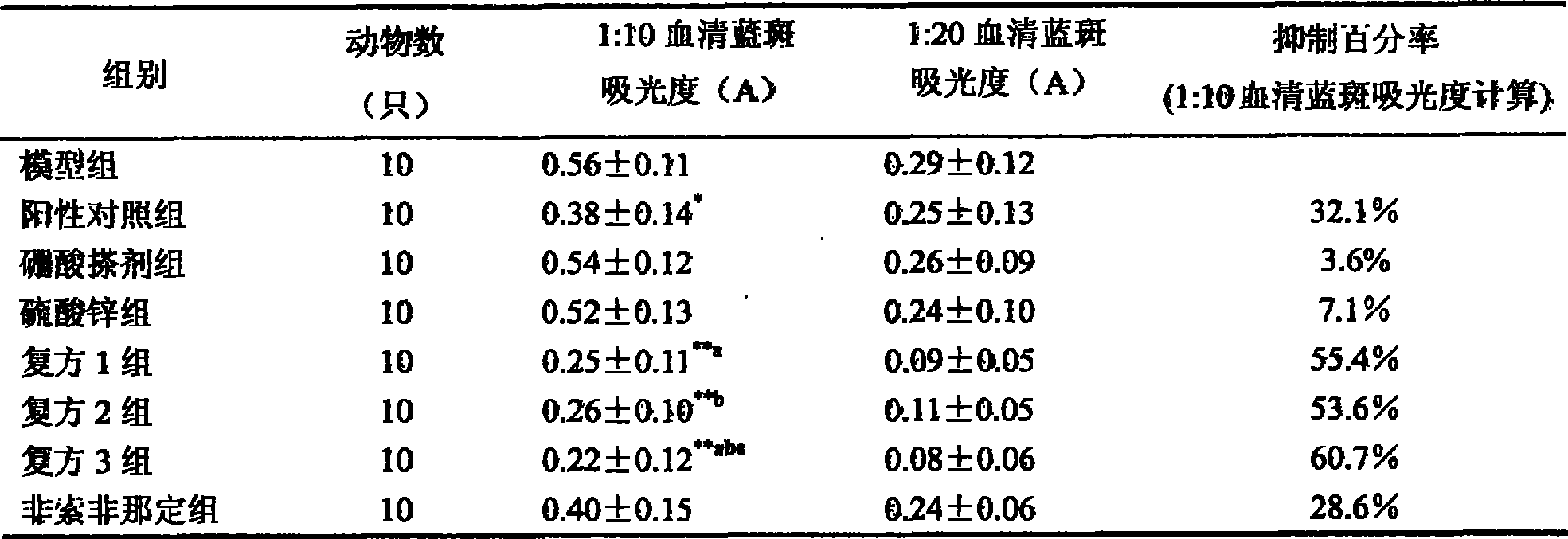
[0017] Example 1 Allogeneic Passive Skin Allergy Test in Rats
[0018] 1. Experimental method:
[0019] 1. Group settings
[0020] Set up (1) model group, (8) positive drug (piyanping) control group, (2) boric acid liniment group (containing boric acid 3%), (3) zinc sulfate liniment group (containing zinc sulfate 5%), (4) Fexofenadine hydrochloride compound group 1 (fexofenadine hydrochloride + boric acid, containing fexofenadine hydrochloride 20mg / ml, boric acid 3%), (5) Fexofenadine hydrochloride compound group 2 (fexofenadine hydrochloride+zinc sulfate, containing fexofenadine hydrochloride 20mg / ml, zinc sulfate 5%), (6) fexofenadine hydrochloride compound 3 groups (fexofenadine hydrochloride+boric acid+ Zinc sulfate, containing fexofenadine hydrochloride 20mg / ml, boric acid 3%, zinc sulfate 5%), (7) fexofenadine hydrochloride liniment group (containing fexofenadine hydrochloride 20mg / ml), a total of 8 groups.
[0021] 2. Operation steps
[0022] Preparation of antiser...
Embodiment 2
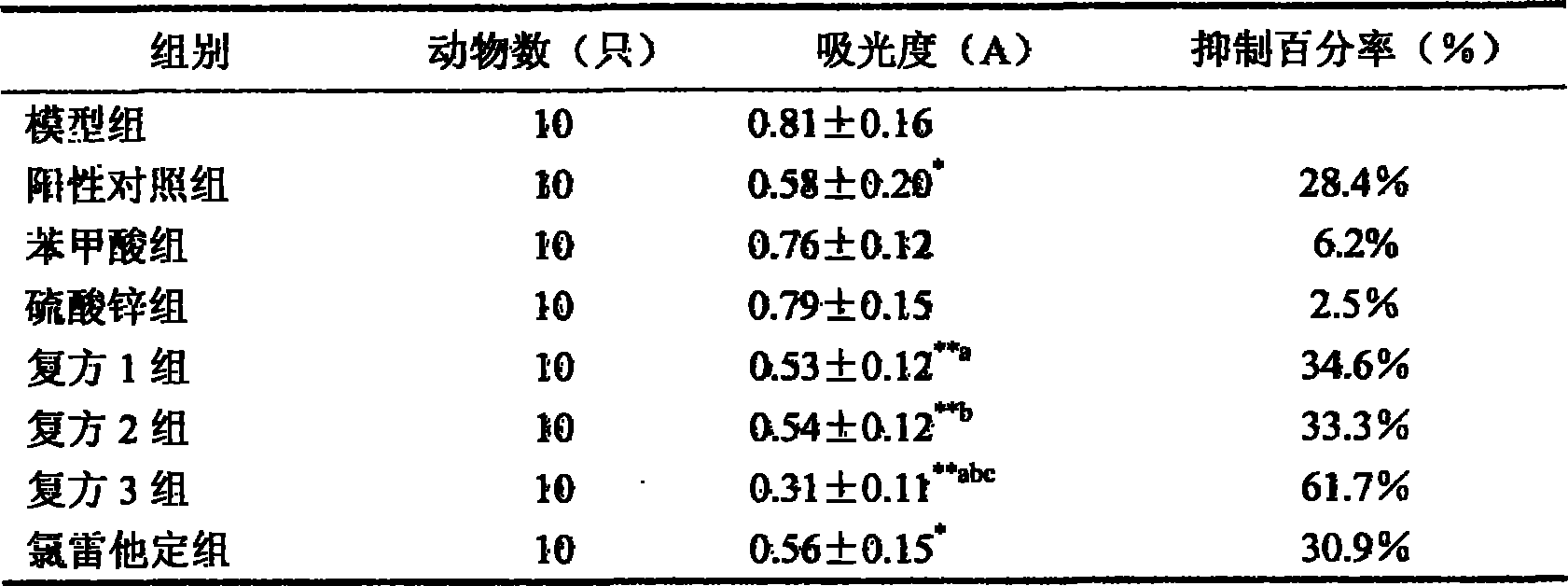
[0033] Example 2 Mouse ear xenogeneic passive cutaneous anaphylaxis (PCA)
[0034] Mouse ear xenogeneic passive cutaneous anaphylaxis is a highly sensitive and reproducible model of type I allergy. The serum of sensitized rats (containing rich IgE antibodies) is intradermally injected into the auricles of normal mice to make them passively sensitized. When the antigen is attacked, the local vascular permeability of the auricle increases, and Evanslan is injected into the auricle. According to the amount of Evans blue infiltrated, it can reflect the degree of skin allergic reaction and the efficacy of antihistamine drugs.
[0035] 1. Experimental method:
[0036] Antiserum preparation: same as Example 1
[0037] 1. Group settings:
[0038] Set up respectively (1) model group, (2) positive drug (piyanping) control group, (3) benzoic acid group (containing benzoic acid 2%), (4) zinc sulfate group (containing zinc sulfate 8%), (5 ) loratadine compound recipe 1 group (loratadine...
Embodiment 3
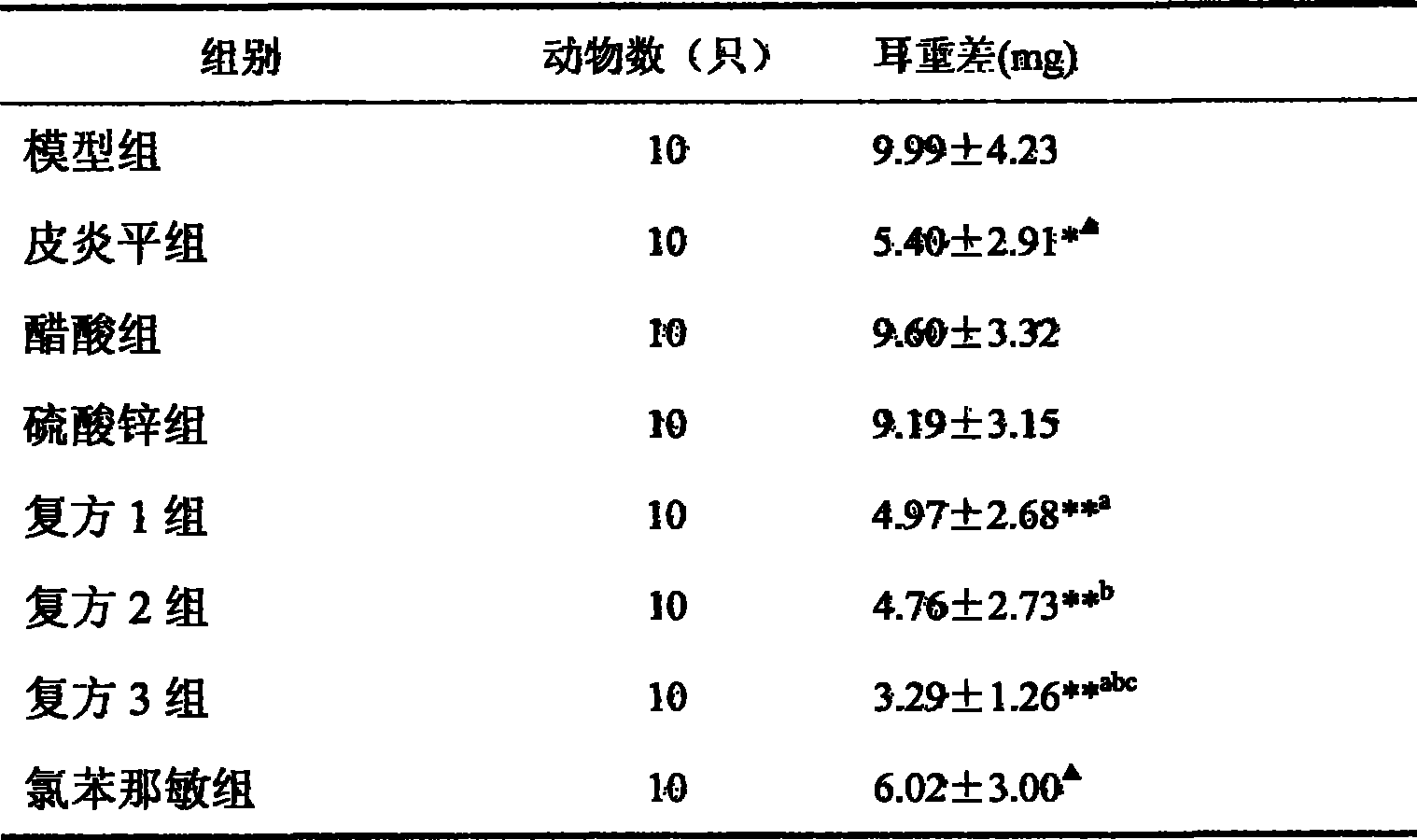
[0049] Example 3 Effect of Chlorpheniramine Compound on Delayed Type Hypersensitivity (DTH) in Mice Induced by Dinitrofluorobenzene (DNFB)
[0050] Delayed type hypersensitivity (DTH) is a reaction dependent on T cells, and its main feature is that the sensitized body has a delayed inflammatory response at the site of antigen attack, and T cells in the DTH reaction are involved in graft rejection and graft versus host disease Therefore, it is necessary to study the influence of drugs on the induction and regulation of DTH response to find new drugs and clarify the mechanism of action.
[0051] DNFB is a hapten. After its dilution is applied to the skin of the abdomen, it combines with skin proteins to form a complete antigen, thereby stimulating T lymphocytes to proliferate into sensitized lymphocytes. After 4-7 days, apply it to the skin of ears or paws to cause local delayed-type allergic reactions, which generally reach the peak at 24-48 hours after antigen attack, so the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com