Novel isosorbide mononitrate micro-porous osmotic pump controlled-release preparation and preparation method thereof
A technology of isosorbide dinitrate and controlled-release preparations, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, cardiovascular system diseases, etc., can solve the problems of incomplete drug release and decreased drug release power, and improve patient Effects of compliance, reduction of terminal residues, and reduction in the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
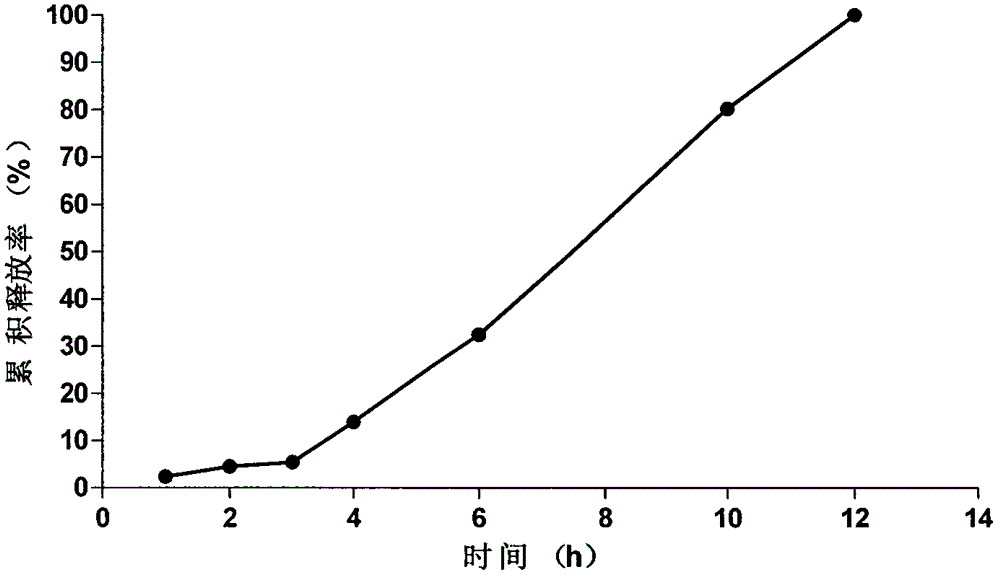
Embodiment 1
[0046] Osmotic active substance delayed-release pellet core prescription (1000 pieces):
[0047] Glucose 10.27g
[0048] Microcrystalline Cellulose 4.40g
[0049] Preparation process of pellet core: glucose and microcrystalline cellulose are respectively passed through 80-mesh sieve, then passed through 60-mesh sieve and mixed evenly, water is used as a binder to make soft material, extruded and spheronized, dried at 40°C for 24 hours, and sieved. Pellets between 24-30 mesh.
[0050] The formula composition of the coating solution of the pellet isolation layer (coating weight gain is 10.0%):
[0051] HPMC 2g
[0052] Ethanol 36ml
[0053] water 4ml
[0054]Coating process of isolation gown: take the pellet core and place it in a fluidized bed, adjust the air inlet temperature to 35°C, and the blast frequency to 30.50HZ, slowly add HPMC to the ethanol water solvent, and use it as an isolation layer coating after being clarified and transparent Use a peristaltic pump to p...
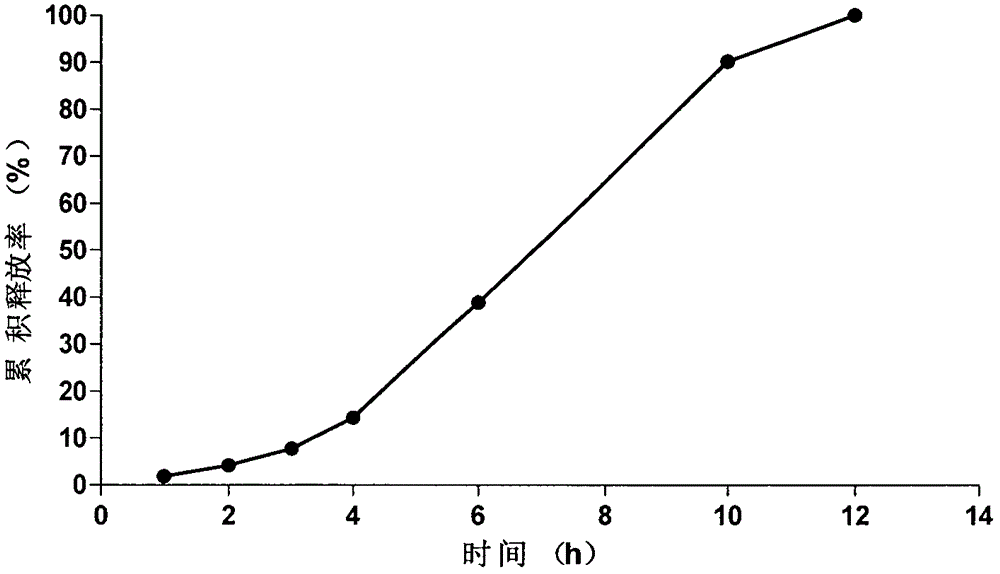
Embodiment 2
[0066] The prescription and preparation process of the osmotically active substance delayed-release pellets are the same as in Example 1, the weight gain of the coating of the isolation layer is 9.5%, and the weight gain of the coating of the controlled release layer is 14.5%.
[0067] Tablet core prescription composition (1000 tablets):
[0068]
[0069] The prescription composition of semi-permeable membrane coating liquid (coating weight gain 4.45%):
[0070]
[0071] The tablet core of the present embodiment is prepared according to the tablet core preparation method of the isosorbide mononitrate of Example 1, and according to the coating solution prescription, the tablet core of the present embodiment is coated by the coating process of Example 1, and solidified to obtain the present embodiment. Novel Isosorbide Mononitrate Microporous Osmotic Pump Formulations of the Examples.
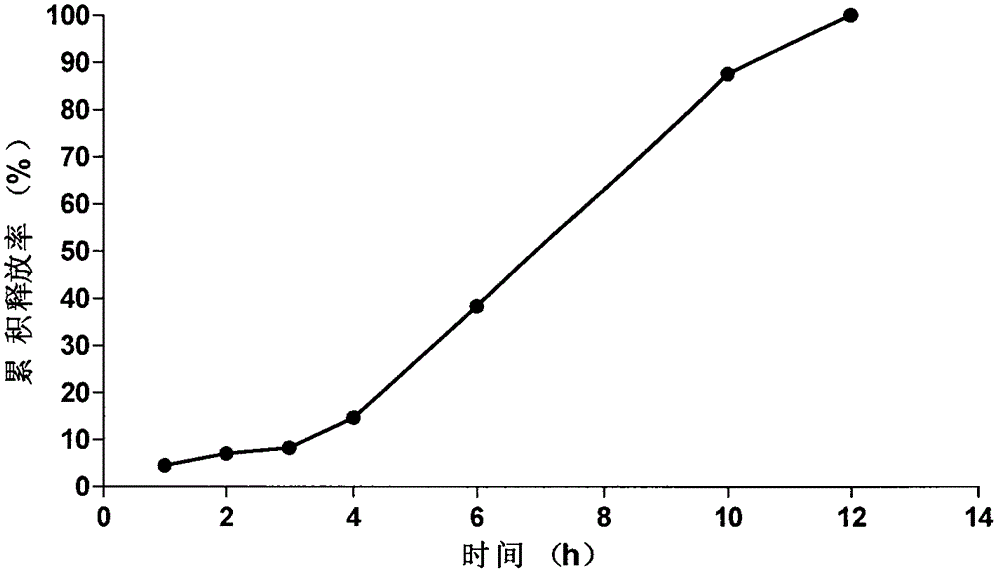
Embodiment 3
[0073] The prescription and preparation process of the osmotically active substance delayed-release pellets are the same as in Example 1, the weight gain of the coating of the isolation layer is 10.5%, and the weight of the coating of the controlled release layer is 15.5%.
[0074] Tablet core prescription composition (1000 tablets):
[0075]
[0076] The prescription composition of semi-permeable membrane coating liquid (coating weight gain 4.55%):
[0077]
[0078] The tablet core of the present embodiment is prepared according to the tablet core preparation method of the isosorbide mononitrate of Example 1, and according to the coating solution prescription, the tablet core of the present embodiment is coated by the coating process of Example 1, and solidified to obtain the present embodiment. Novel Isosorbide Mononitrate Microporous Osmotic Pump Formulations of the Examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com