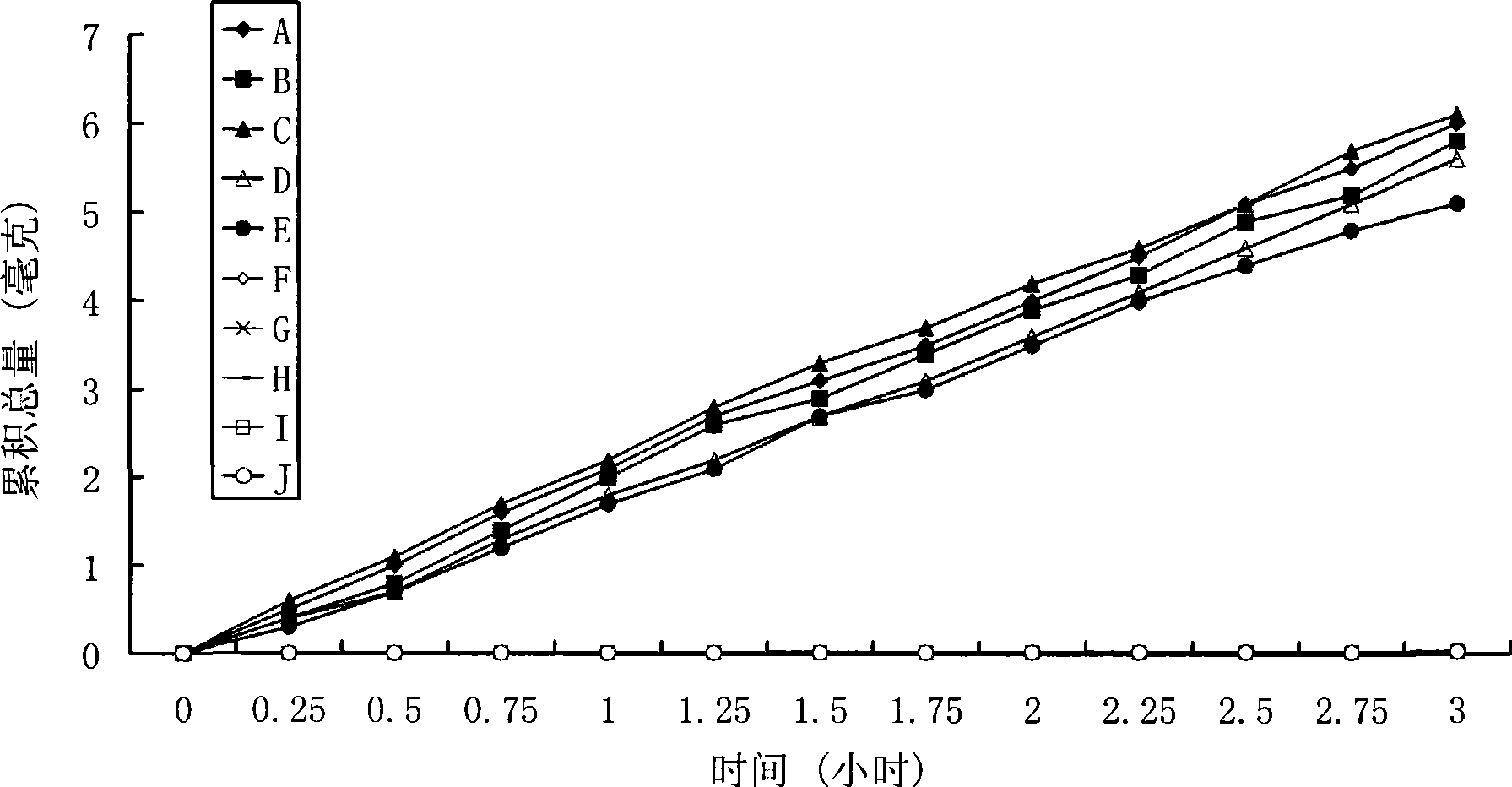
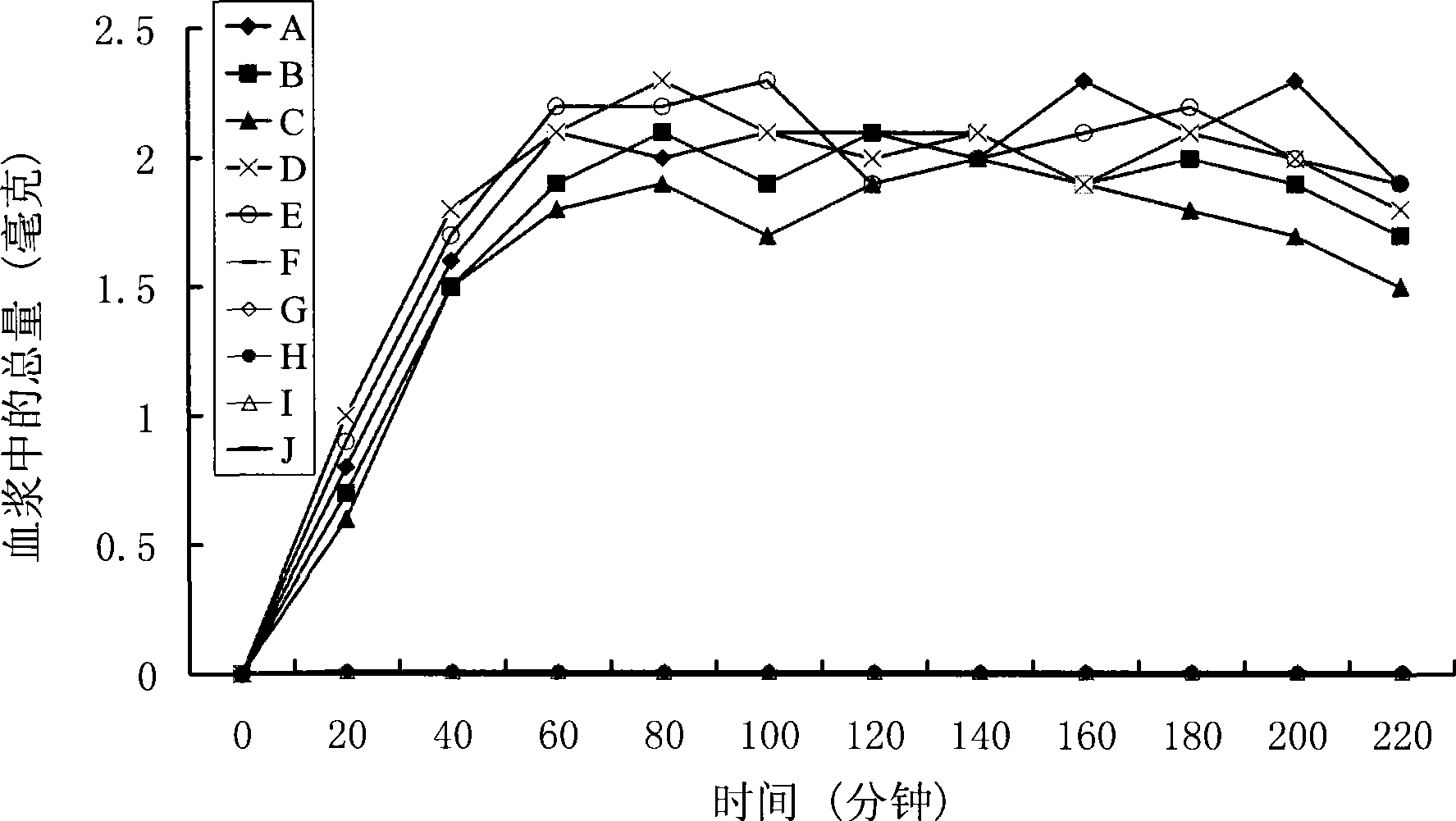
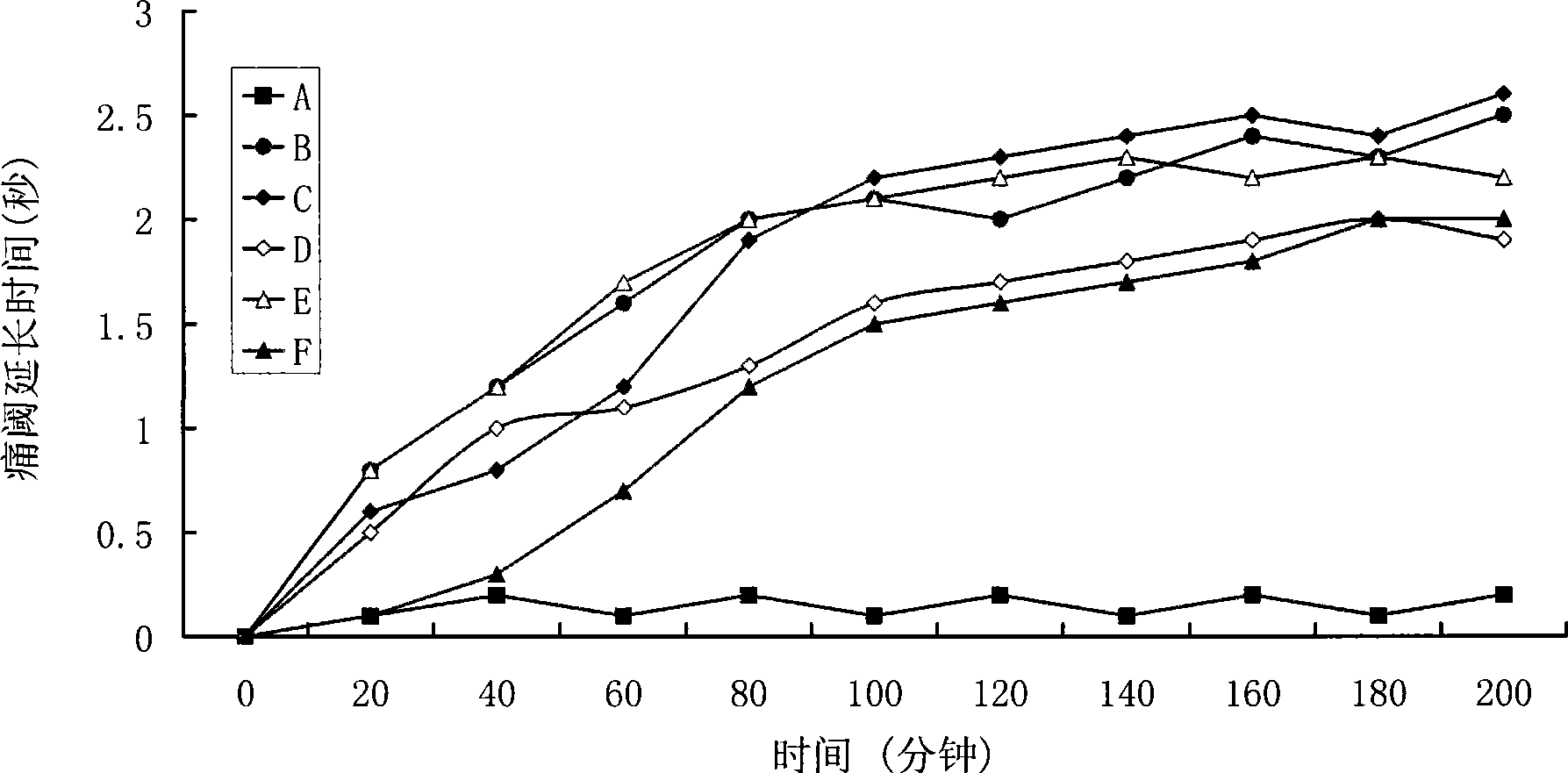
Positively charged water-soluble prodrugs of n-arylanthranilic acids with very fast skin penetration rate
An aryl and alkyl technology, applied in the field of prodrugs of positively charged water-soluble aryl anthranilic acid with fast skin penetration speed, can solve problems such as slow skin penetration speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0056] The synthetic method of N-diethylaminoethyl-2-[(2,6-dichloro-3-methylphenyl)amino]benzamide acetate
[0057] 29.6 g (0.1 mol) of 2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid were dissolved in 300 ml of chloroform. 20.6 g of N,N'-dicyclohexylcarboimide was added to the reaction mixture. 11.7 g of N,N-diethylaminoethylamine was added to the reaction mixture. The reaction mixture was stirred at room temperature for 3 hours. The solids were removed by filtration. The chloroform solution was washed twice with 100 ml of 5% aqueous sodium bicarbonate solution and three times with 100 ml of water. The organic solution was dried over anhydrous sodium sulfate. Sodium sulfate was removed by filtration. 6 g of acetic acid was stirred into the reaction mixture. Hexane (200ml) was added. The solid product was collected by filtration. After drying, 39 g of the hygroscopic target product were obtained with a yield of 85.8%. Solubility in water: 400mg / ml; Elemental analys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com