Methods for determining plasma free drug concentration by direct measurement of binding affinity of protease inhibitors to plasma proteins
a protease inhibitor and plasma protein technology, applied in the field of isothermal titration calorimetry analysis of the binding affinity of protease inhibitors to plasma proteins, can solve the problems of % change in free drug concentration, toxic response, significant changes in clinical respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Thermodynamic Equilibrium Binding Assay Using ITC
[0049] Binding experiments were run using Isothermal Titration Calorimetry (ITC) to measure bound and unbound protease inhibitors.
[0050] Sample preparation: Solutions of AAG and its variants for ITC experiments were made up in 20 mM sodium borate buffer, pH7.4. All compounds were prepared as stock solution of 20 mM or 25 mM in DMSO and thereafter diluted to desired concentration in the same buffer. For all ITC experiments, the percentage of DMSQ is below 1%.
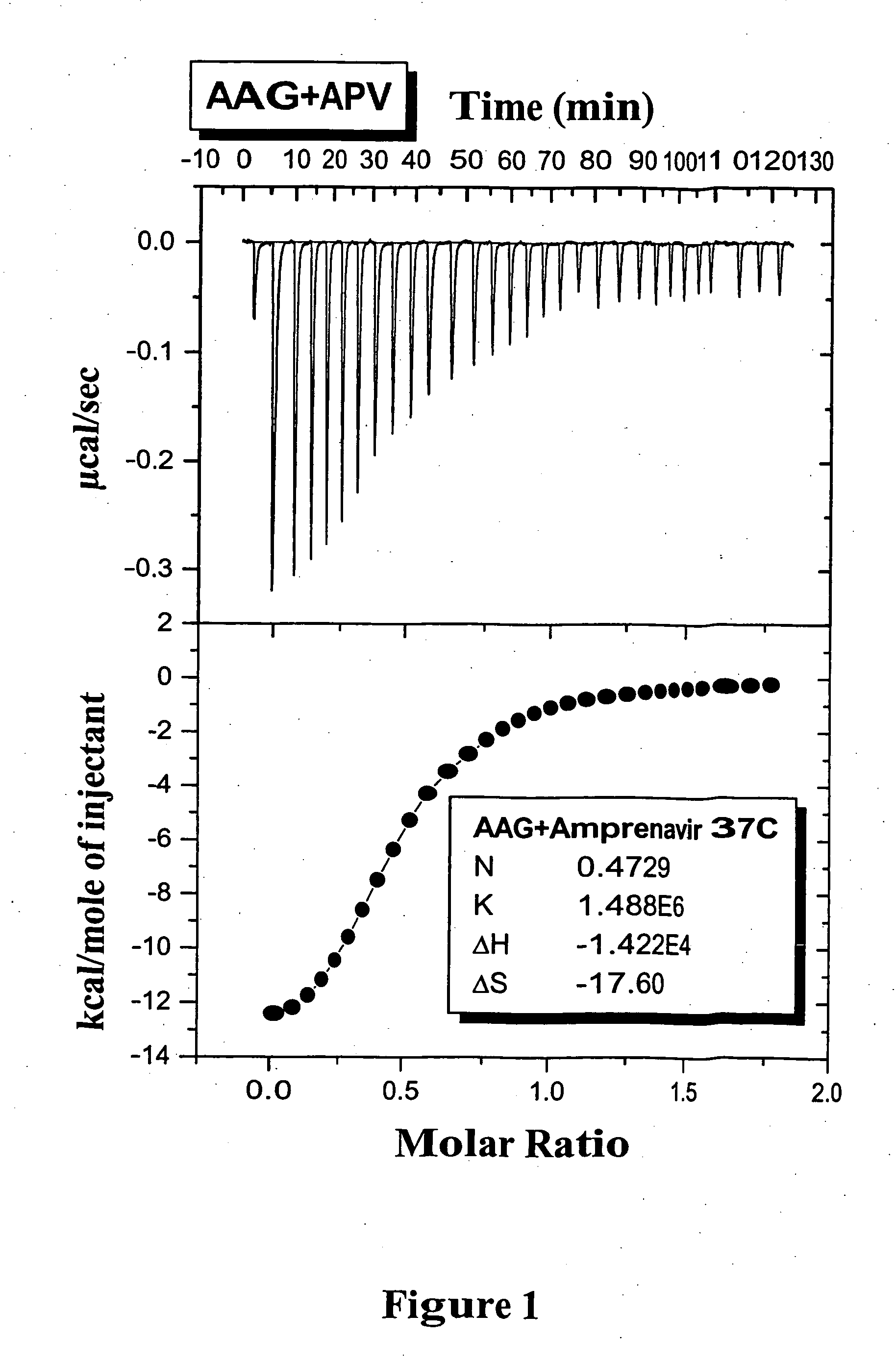
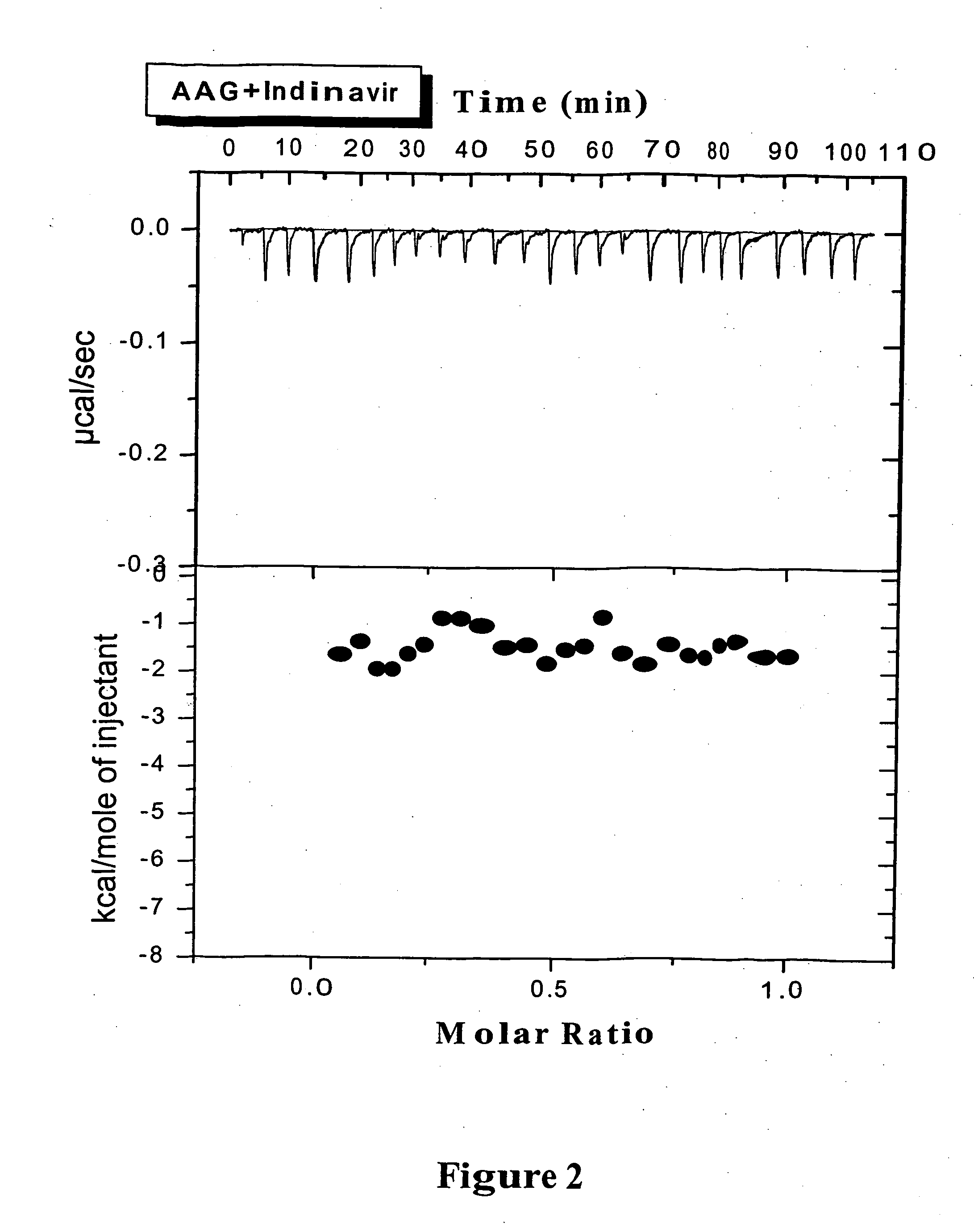
[0051] ITC Experiments: The isothermal titration measurements of the binding of various PIs to AAG can be typically carried out at 37° C. using a VP-ITC titration calorimeter (MicroCal, Northampton, Mass.). The instrument was electrically calibrated by means of a standard electric pulse as recommended by the manufacturer. For the PIs binding to AAG, solutions of PI (80˜100 μM) were used to titrate AAG (10˜12 μM). A 300 μL syringe was used for the titrant, mixing was effected by ...
example 2
Ka can be Utilized to Predict the Effect of AAG on EC50 Value in Cell-Based Antiviral Assays
[0054] In order to determine whether there is a strong correlation between the loss of activity of PIs in presence of AAG and their binding affinities measured directly by ITC, the antiviral EC50 of these PIs in the presence of 1 mg / mL of AAG from their binding constants Ka with AAG and their EC50 in the absence of AAG was calculated according to Equation 4. FIG. 3 shows the calculated and experimental EC50 of 7 PIs studied here in the presence of 1 mg / mL AAG and in the absence of AAG. As discussed above, the addition of 1 mg / mL AAG in the cell culture markedly decreased the EC50 of PIs except IDV, herein we consider the presence of AAG has no effect on EC50 of IDV. Comparison of the calculated and experimental EC50 of these PIs in the presence of AAG revealed that the calculated and experimental values were highly consistent (FIG. 3). FIG. 4 shows the calculated EC50 versus experimental EC5...
example 3
Comparative Evaluation of the In Vivo Antiviral Efficacy of PIs in the Presence of AAG
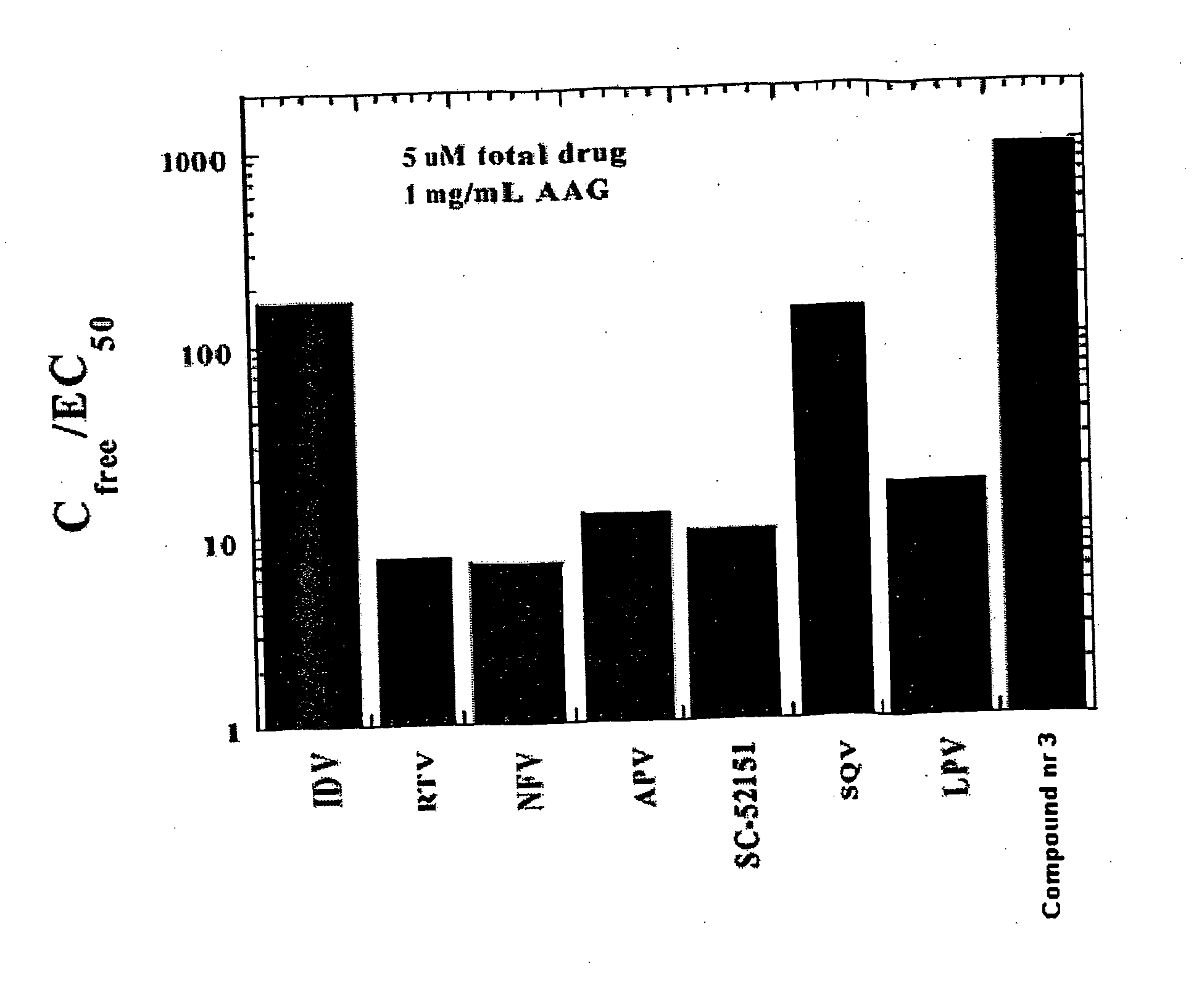
[0055] The evaluation of the relative in vivo efficacy of PIs in human plasma from their EC50 (Equation 5) is shown in FIG. 5. The validation of the correct equation was confirmed using published data for three PIs monotherapy trials.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| binding affinity | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com