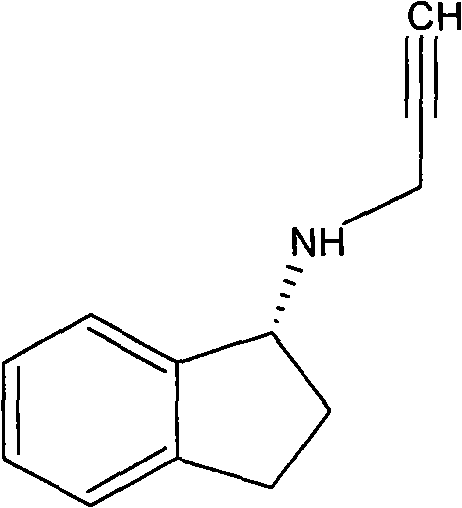
Slow release long-acting rasagiline transdermal patch with high bioavailability and preparation method thereof
A transdermal patch and utilization technology, applied in the direction of non-active ingredient medical preparations, sheet delivery, drug combination, etc., can solve the problems of undisclosed prescriptions and preparation methods, and avoid the first-pass effect of the liver, Smooth absorption and the effect of reducing blood pressure response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
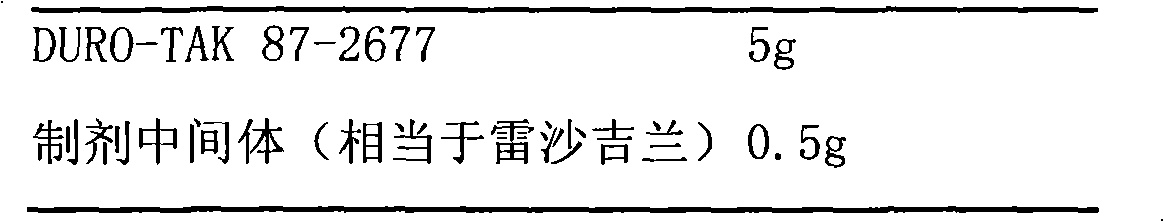
[0059] Patches based on DURO-TAK87-2677 pressure sensitive adhesive
[0060] prescription:
[0061]
[0062] Add the preparation intermediate to DURO-TAK87-2677, stir and mix well, coat 0.25mm thick on a 75μm thick translucent polyester film, dry at 60°C for 30 minutes, evaporate the organic solvent, and coat 75μm thick A polyethylene backing layer is available. The patch has a measured content of 923 μg / cm 2 . The patch was cut into a size containing 5 mg of rasagiline for human pharmacokinetic research.
Embodiment 2
[0064] Patches based on DURO-TAK87-4098 pressure-sensitive adhesive
[0065] prescription:
[0066]
[0067] Add the preparation intermediate and butyl hydroxytoluene (butyl hydroxytoluene is used as an antioxidant) to DURO-TAK 87-4098, stir and mix well, and coat 0.25 mm thick on a 75 μm thick translucent polyester film, Dry at 60°C for 30 minutes, evaporate the organic solvent, and cover with a polyethylene backing layer with a thickness of 75 μm. The patch measured content of 1080μg / cm 2 . The patch was cut into a size containing 5 mg of rasagiline for human pharmacokinetic research.
Embodiment 3
[0069] Patches based on silicone BIO-PSA7-4302 pressure-sensitive adhesive
[0070] prescription:
[0071]
[0072] Add rasagiline free base into BIO-PSA7-4302, stir and mix evenly, coat 0.2mm thick on a 75μm thick translucent polyester film, dry at 60°C for 30 minutes, evaporate the organic solvent, cover A polyethylene backing layer with a thickness of 75 μm is applied. The patch measured a content of 325 μg / cm 2 . The patch was cut into a size containing 5 mg of rasagiline for human pharmacokinetic research.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com