All-trans retinoic acid liposome preparation and preparation and application thereof
A technology of all-trans retinoic acid and liposome preparations, which is applied in the direction of liposome delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of all-trans retinoic acid liposomes Unsatisfactory drug loading and in vivo stability, to achieve good in vivo stability, high drug loading, and high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
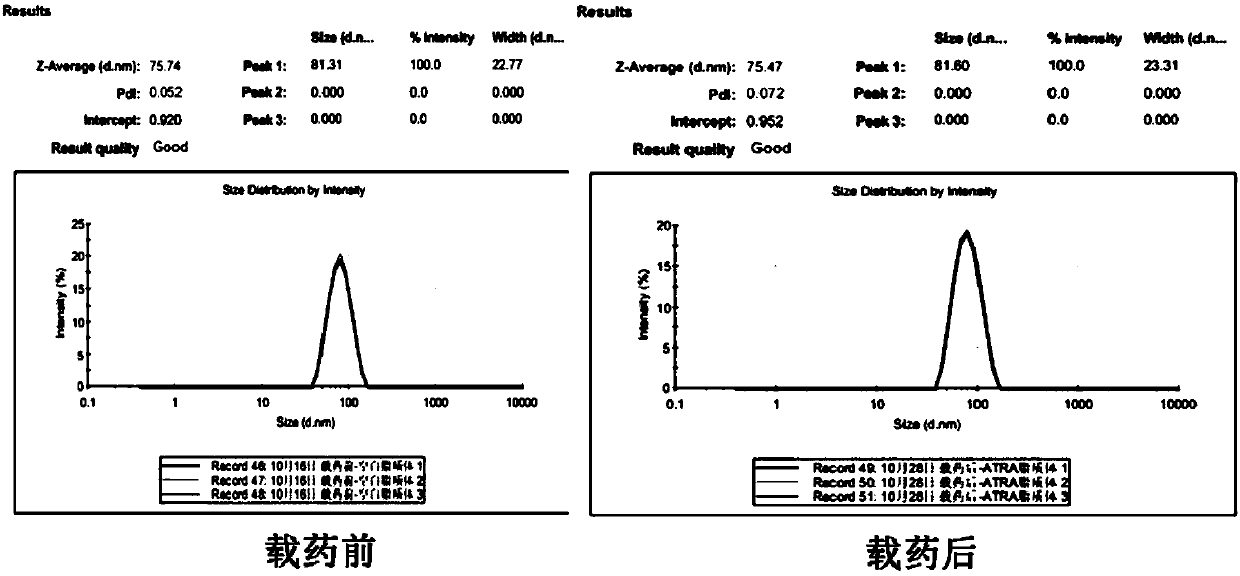
[0074] Preparation and identification of embodiment 1 all-trans retinoic acid liposome
[0075] Hydrogenated soy lecithin (HSPC) was purchased from NOF Corporation, and pegylated phospholipids: distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG2000) and cholesterol were purchased from Avanti PolarLipids, USA; Trans-retinoic acid was purchased from Sigma, USA.
[0076] One, the preparation of all-trans retinoic acid liposome
[0077] (1) Weigh 121.742 grams of hydrogenated soybean lecithin (HSPC, molecular weight 783.8), 38.22 grams of distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG2000) and 40.04 grams of cholesterol, dissolve them with 1.6 milliliters of ethanol, and Water bath in a water bath at 70 degrees Celsius to dissolve and mix to obtain an ethanol mixture;
[0078] (2) Add 6.4 ml of the ethanol mixture obtained in step (1) to calcium acetate buffer (pH 9.0, which consists of 200 mM calcium acetate and water), and place in a w...
Embodiment 2
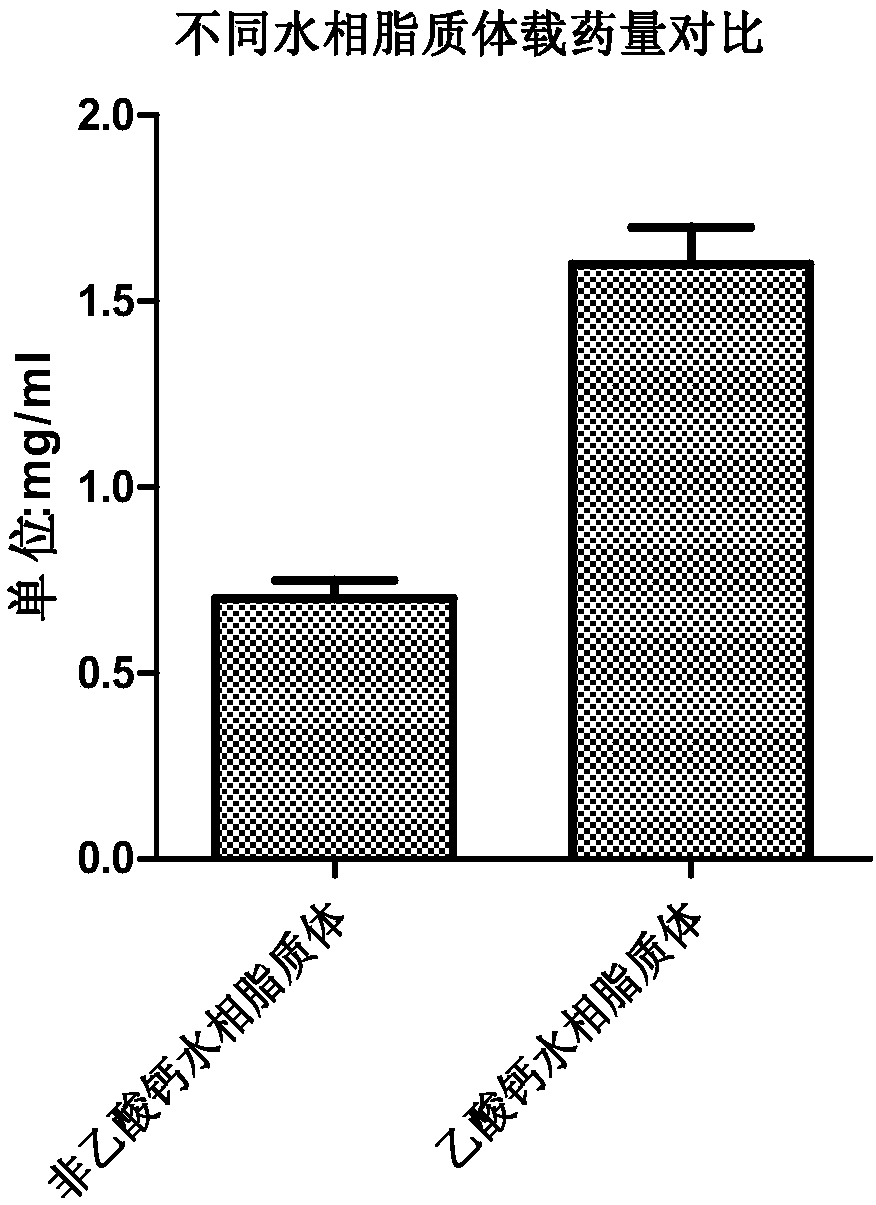
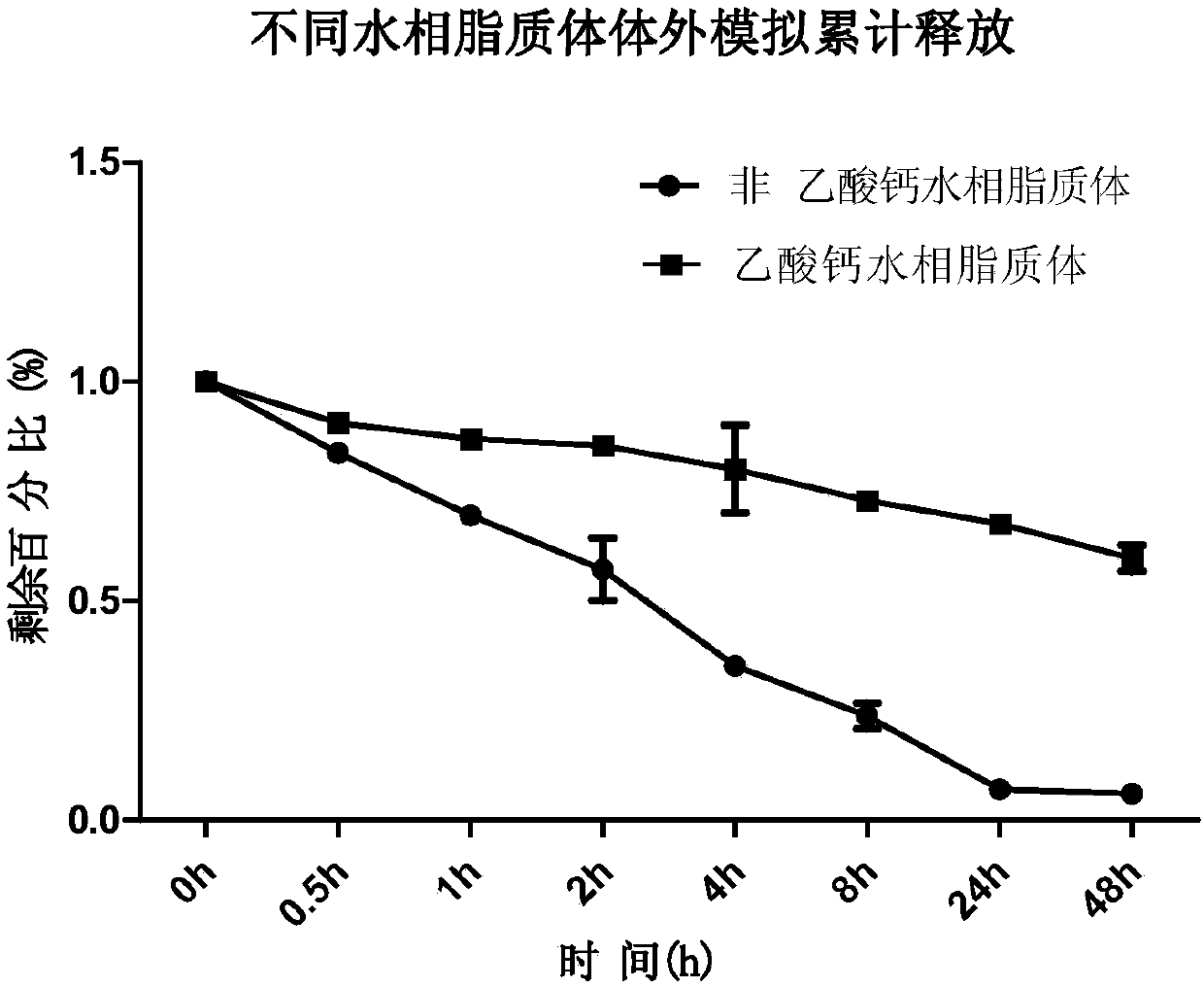
[0094] Embodiment 2, active drug loading prepares all-trans retinoic acid liposome and passive drug loading prepares all-trans retinoic acid liposome comparison
[0095] 1. Preparation of all-trans retinoic acid liposomes by active drug loading method and passive drug loading method
[0096] 1. Preparation of all-trans retinoic acid liposomes by active drug loading method
[0097] (1) Weigh 121.742 grams of hydrogenated soybean lecithin (HSPC, molecular weight 783.8), 38.22 grams of distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG2000) and 40.04 grams of cholesterol, dissolve them with 1.6 milliliters of ethanol, and Water bath in a water bath at 70 degrees Celsius to dissolve and mix to obtain an ethanol mixture;
[0098] (2) Add 6.4 ml of the ethanol mixture obtained in step (1) to calcium acetate buffer (pH 9.0, which consists of 200 mM calcium acetate and water), and place in a water bath at 70 degrees Celsius for 30 minutes to obtain liposome vesicle...
Embodiment 3
[0116] Example 3. All-trans retinoic acid liposomes induce differentiation of tumor myeloid suppressor cells in vitro
[0117] 1. Tumor model establishment in Balb / c mice
[0118] (1) When CT-26 cells were cultured to the logarithmic growth phase, they were digested with trypsin, and the digested cells were collected and centrifuged in a centrifuge at a speed of 300g for 5 minutes, the supernatant was discarded, and the cells were resuspended with sterile PBS. Count the cells and adjust the cell concentration to 1*10 7 cells / ml;
[0119] (2) Purchase 6-week-old Balb / c white mice, shave the fur on the side of the subcutaneous inoculation in advance, inject 200 μl of 4% chloral hydrate intraperitoneally to anesthetize the mouse, and inject it subcutaneously in the right underarm for digestion The CT-26 suspension, the cell seeding volume is 5*10 5 -1*10 6 / only, continue to raise after inoculation;
[0120] (3) After feeding for about 2-3 weeks, use a vernier caliper to me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com