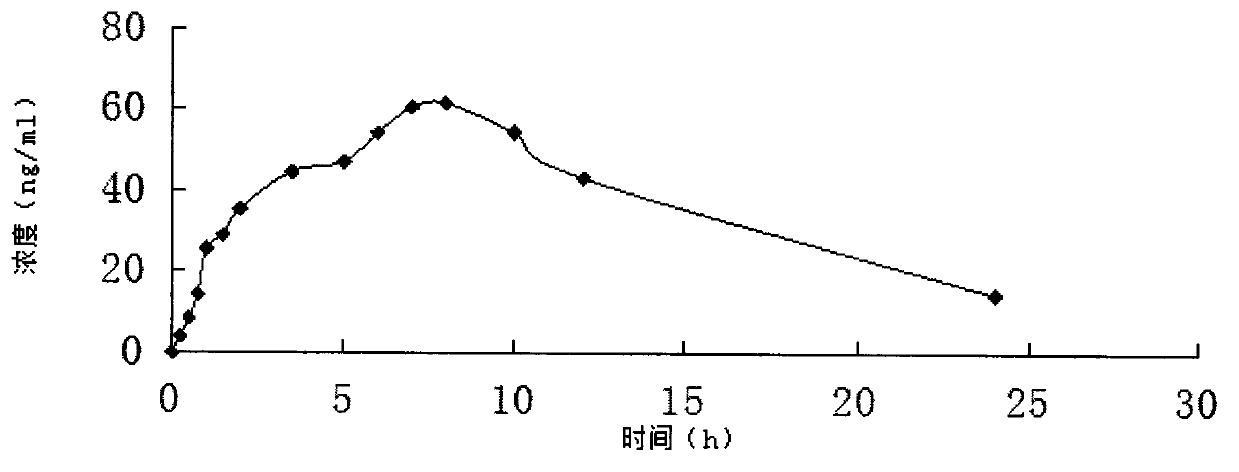
Piribedil sustained-release tablet and preparation method thereof
A technology of piribedil and sustained-release tablets, which is applied in the field of medicine, can solve problems such as poor effect, poor stability, and decreased drug efficacy, and achieve the effects of avoiding motor complications, improving stability, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] Meanwhile, in a typical embodiment of the present invention, the preparation method of the above-mentioned piribedil sustained-release tablets includes the following preparation steps in order: (1) mixing the piribedil, framework material and filler after crushing uniform; (2) adding binder to make soft material, granulating, and drying; (3) adding lubricant to the dried granules, mixing, and tableting to obtain the piribedil sustained-release tablet. The method is simple and easy to implement, and the prepared piribedil sustained-release tablet has good dissolution rate and stability.
[0020] The specific implementation of each step in the preparation method of the above-mentioned piribedil sustained-release tablet will be given below.
[0021] Preferably, in the preparation method of the above-mentioned piribedil sustained-release tablets, the preparation step (1) further includes: pulverizing each raw material, passing through a 60-80 mesh sieve, and setting aside; ...
Embodiment 1
[0025] Raw materials and equipment:
[0026]
[0027] Preparation Process:
[0028] (1) pass the raw and auxiliary materials through an 80-mesh sieve, and set aside;
[0029] (2) Weigh the piribedil of the prescribed amount, the skeleton material, and the filler and divide them into 20 equal parts respectively, add and mix them successively, and vibrate for 10 minutes at a frequency of 10 times / s;
[0030] (3) Add an appropriate amount of binder to make a soft material, pass through a 26-mesh sieve to granulate, and dry in a 60-degree oven for 1 hour;
[0031] (4) Pass through a 26-mesh sieve for granulation, add an appropriate amount of lubricant, mix evenly, compress into tablets, and check. The dissolution data are shown in Table 1.
[0032] The release test results of the piribedil sustained-release tablets prepared in Table 1 Example 1
[0033] Labeled cumulative release %
Embodiment 2
[0035] Raw materials and ratio:
[0036]
[0037] Preparation Process:
[0038] (1) pass the raw and auxiliary materials through a 60-mesh sieve, and set aside;
[0039] (2) Weighing the piribedil of the prescribed amount, the skeleton material, and the filler are divided into 10 equal parts respectively, added and mixed successively, and shaken for 30 minutes at a frequency of 20 times / s;
[0040] (3) Add an appropriate amount of binder to make a soft material, pass through a 25-mesh sieve to granulate, and dry in an oven at 50 degrees for 1 hour;
[0041] (4) Pass through a 25-mesh sieve for granulation, add an appropriate amount of lubricant, mix evenly, compress into tablets, and check. The dissolution data are shown in Table 2.
[0042] The dissolution test result of the piribedil sustained-release tablet prepared by the embodiment 2 of table 2
[0043] Labeled cumulative release %
[0044] 2#
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com