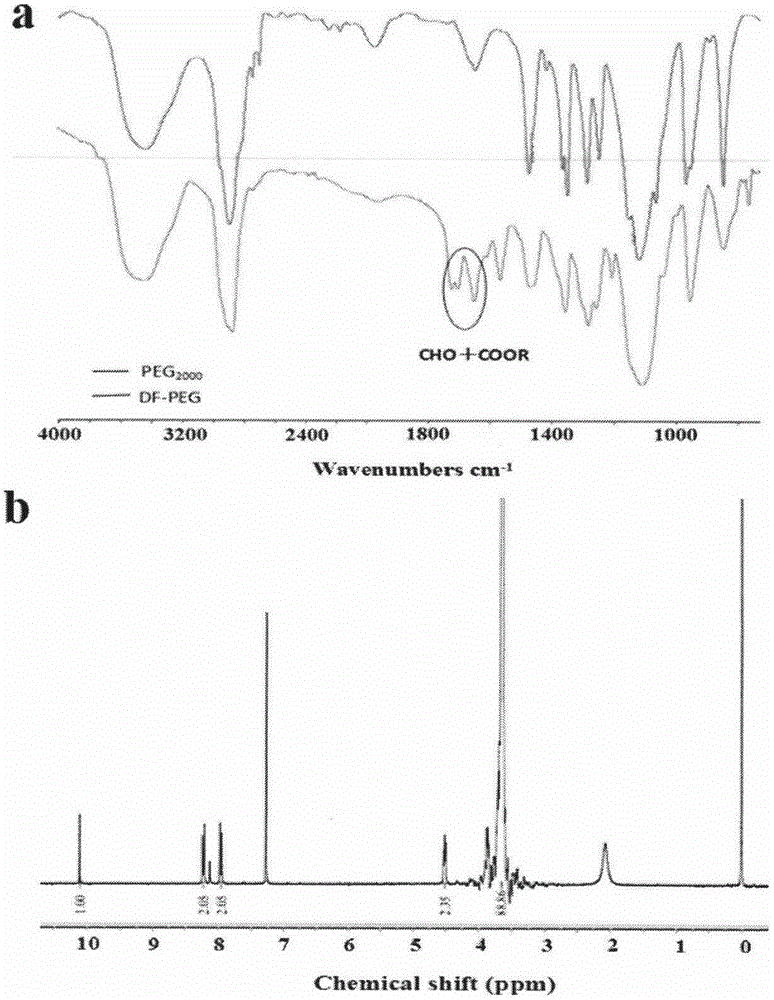
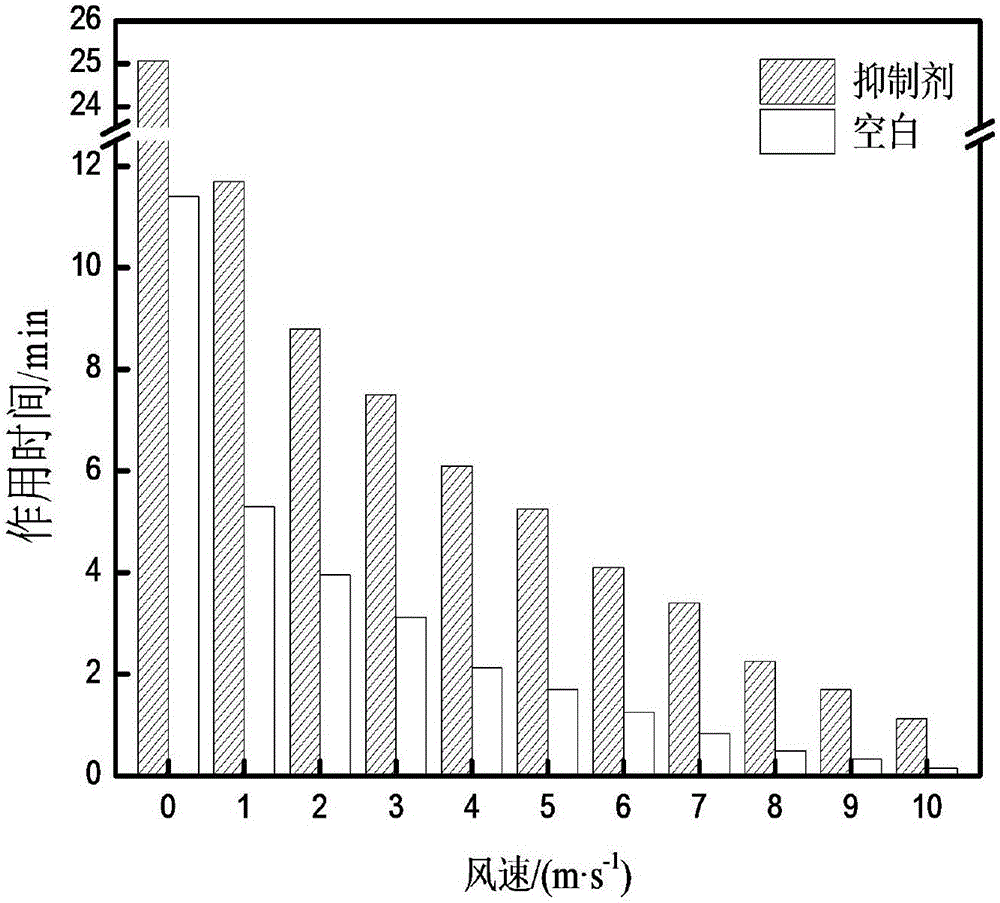
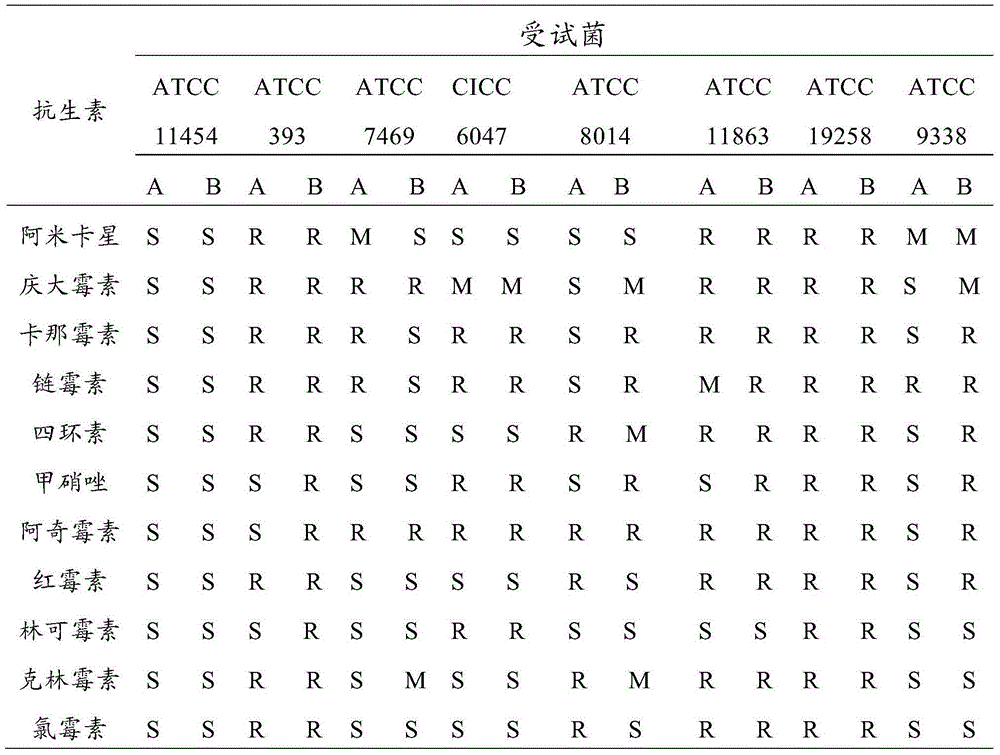
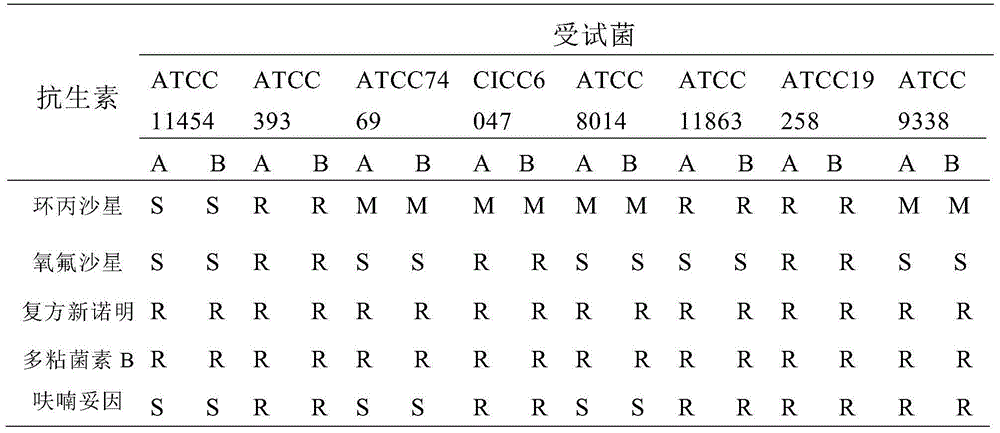
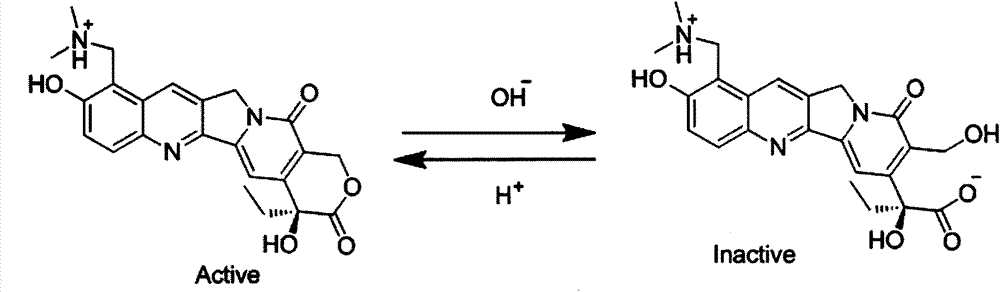
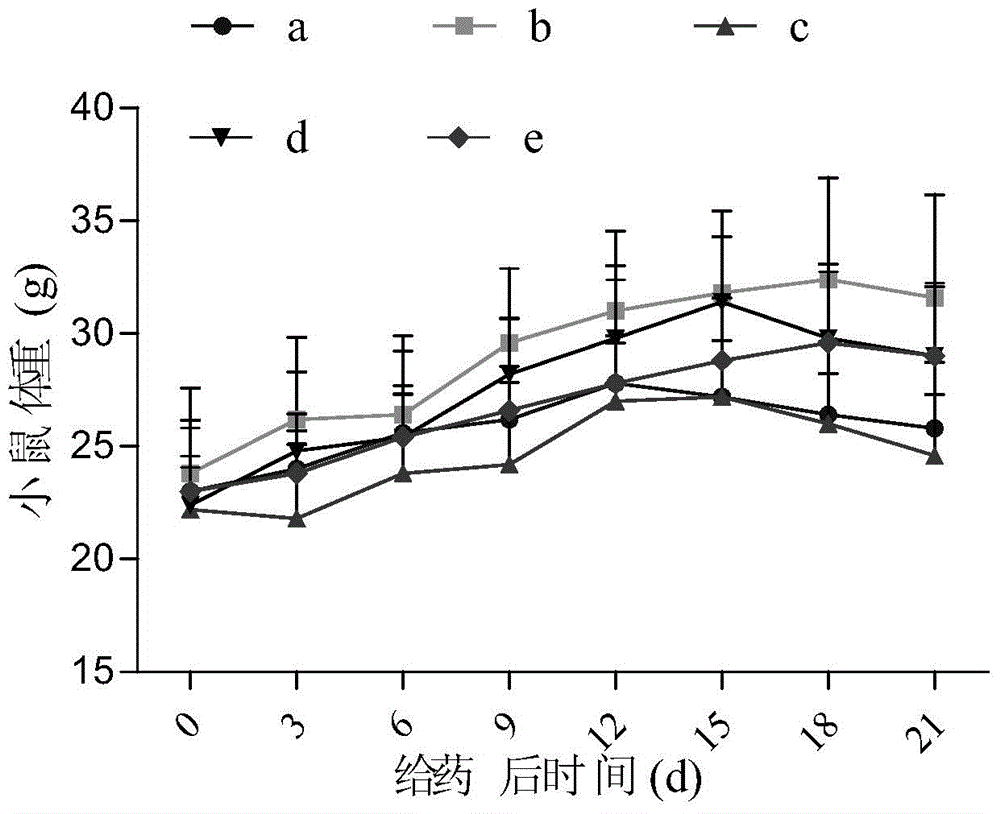
Patents
Literature
32 results about "Beta-glycerophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inducing method and inducing culture medium for differentiation of bone marrow mesenchymal stem cells into osteoblasts in vitro
InactiveCN103667182AFully demonstrate the differentiation abilityConvenient source of serumSkeletal/connective tissue cellsPenicillinCulture fluid
The invention provides an inducing method and inducing culture medium for differentiation of bone marrow mesenchymal stem cells into osteoblasts in vitro. The inducing culture medium is composed of 1*10<-8> mol / L of dexamethasone, 50 mu mol / L of ascorbic acid and 10 mmol / L of sodium beta-glycerophosphate; and solvent is a supernatant of a sclerite complete culture medium and comprises 10% of fetal calf serum, 100 U / mL of penicillin, 100 mg / L of streptomycin, a mixture of DMEM culture fluid and F12 culture fluid and multiple growth factors secreted by bone cells in the sclerite culture process. According to the invention, bone marrow mesenchymal stem cells of a mouse are purified by replacing the cell culture fluid through an adherent cell passage method, the obtained cells of the first generation are induced, and the supernatant of the sclerite complete culture medium cultured for 72-96 hours is used as the solvent of osteoblast differentiation inducer, thereby obviously improving the in vitro osteogenic differentiation efficiency of bone marrow mesenchymal stem cells.
Owner:HUZHOU CENT HOSPITAL
Doxorubicin hydrochloride loaded temperature-sensitive self-healing hydrogel and preparation method thereof
InactiveCN105520906AReduce releaseAvoid sudden releaseOrganic active ingredientsAerosol deliverySelf-healingSide effect
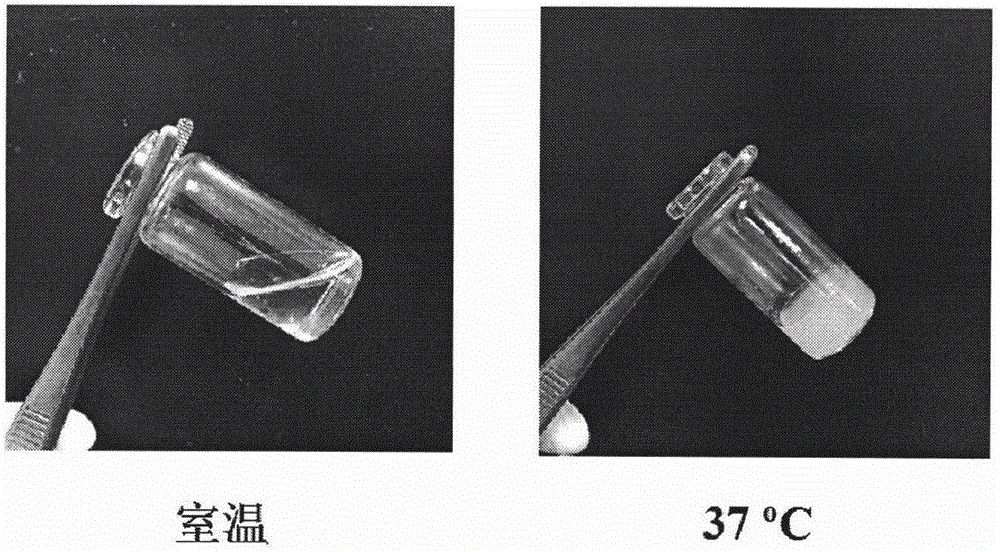
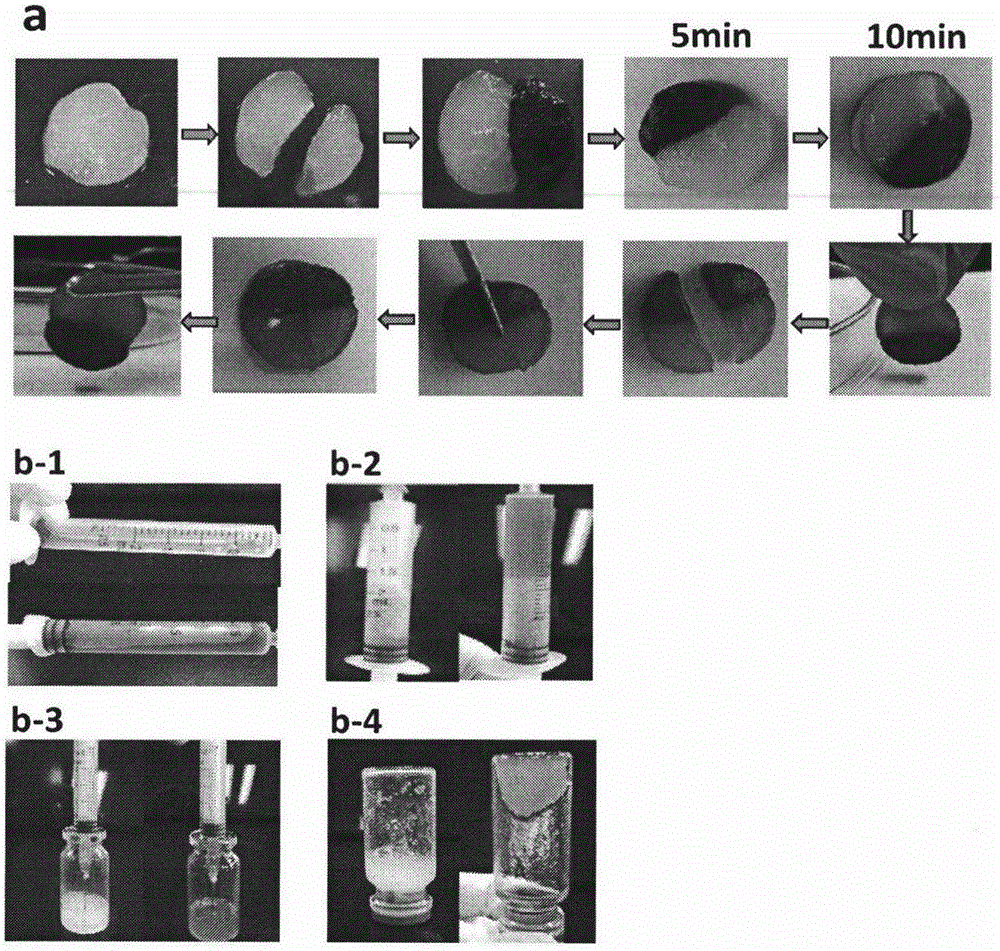
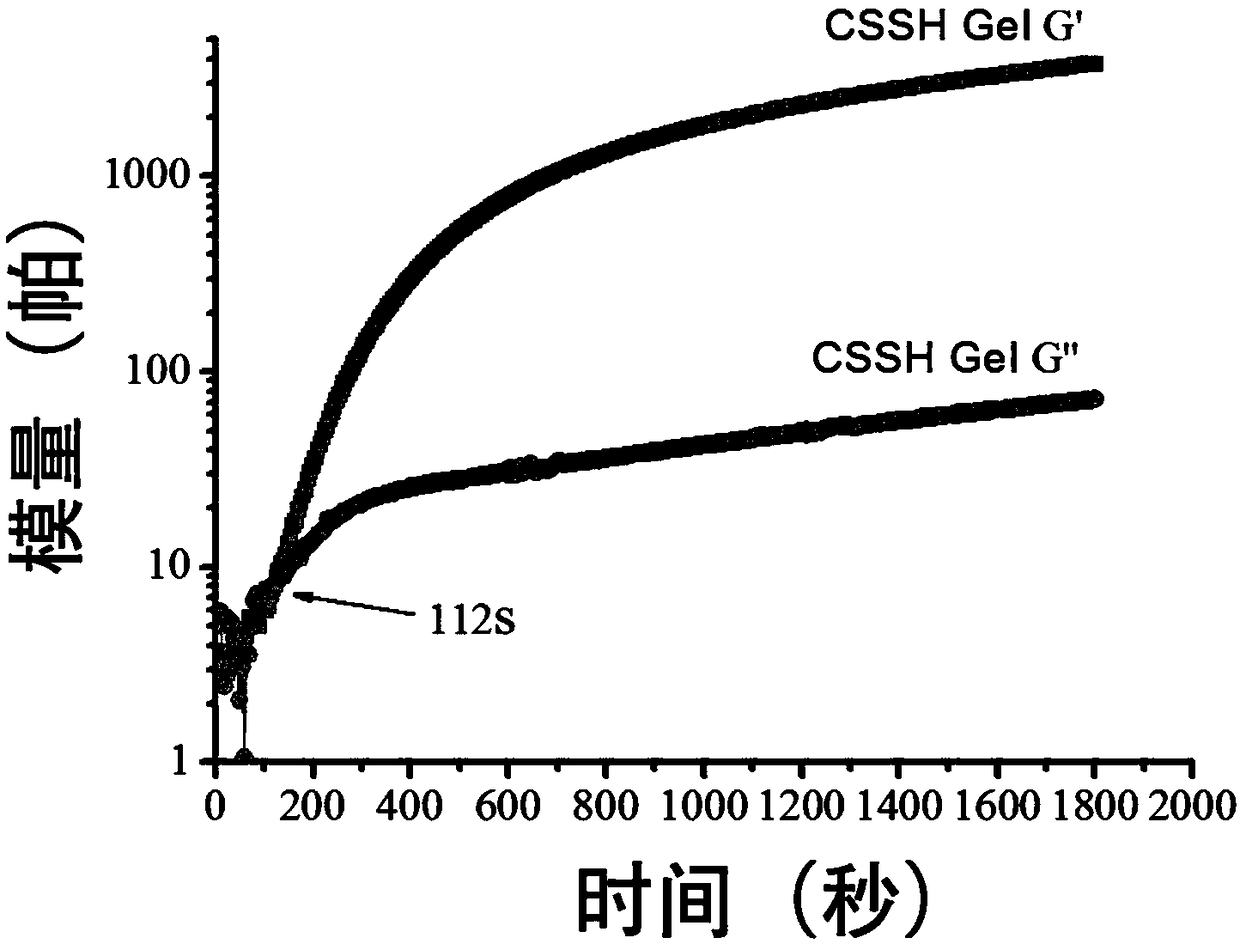
The invention relates to a doxorubicin hydrochloride loaded temperature-sensitive self-healing hydrogel and a preparation method thereof. The doxorubicin hydrochloride loaded temperature-sensitive self-healing hydrogel comprises chitosan, sodium beta-glycerophosphate, and polyethylene glycol with two ends respectively carrying a benzaldehyde group. The preparation method comprises the following steps: forming an imide bond with a self-healing performance through using a reaction of an amino group in the structure of chitosan and the benzaldehyde groups at two ends of the polyethylene glycol, combining the imide bond with chitosan / sodium beta-glycerophosphate temperature-sensitive gel, and loading doxorubicin hydrochloride into the obtained material through physical clathration to prepare the novel temperature-sensitive self-healing hydrogel administration system. The administration system is a liquid at room temperature, and rapidly gelates at a human body temperature after being injected to a tumor position in order to form a medicine reservoir ; and compared with traditional in situ gels, the temperature-sensitive self-healing hydrogel has the advantages of strong mechanical strength, realization of rapid self restoration under the action of outside force or tissue damages to avoid burst release of a medicine, further delay of release of the medicine and prolongation of the detention time of the medicine in the tumor position, medicine effect enhancement and reduction of toxic and side effects.
Owner:CHINA PHARM UNIV
Injectable chitosan composite hydrogel capable of promoting myocardium repair and preparation method of injectable chitosan composite hydrogel
The invention discloses injectable chitosan / keratin composite hydrogel capable of promoting myocardium repair and a preparation method of the hydrogel. The hydrogel consists of four solutions, namely chitosan, sodium beta-glycerophosphate, genipin and keratin according to a volume ratio of 10: (1-5): (1-3): (0.1-0.5). The preparation method comprises the following steps: 1) extracting keratin from human fairs by using peracetic acid and Tris alkaline; 2) adjusting the pH value of a water-soluble chitosan solution by using sodium beta-glycerophosphate so as to be close to a microenvironment in a human body; 3) introducing genipin as a crosslinking agent, and controlling the gelling time of a hydrogel solution by changing the use amount of the genipin so as to achieve the purpose that the mixed solution can be rapidly gelled in situ after being injected into an organism. Experiments prove that the composite hydrogel has favorable biocompatibility and has the capability of remarkably promoting the growth of myocardial cells; the hydrogel is wide in sources of materials and low in cost and has a broad application prospect in the field of tissue engineering repair.
Owner:SOUTHEAST UNIV
Preparation method and application of in-situ injection molded thiolated polysaccharide-based hydrogel and drug carrier thereof
ActiveCN109432496ARich sourcesSimple and fast operationPharmaceutical delivery mechanismProsthesisHalloysiteBeta-glycerophosphate
The invention belongs to the field of tissue engineering scaffolds, and discloses a preparation method and application of an in-situ injection molded thiolated polysaccharide-based hydrogel and a drugcarrier thereof. The in-situ injection molded thiolated polysaccharide-based hydrogel is prepared from 3-6%(w / v) of thiolated polysaccharide and 10-29% (w / v) of sodium beta-glycerophosphate, whereinthe thiolated polysaccharide can coat one or more of drug-carrying liposomes, drug-carrying thiolated halloysite or polypeptides to form the in-situ injection molded thiolated polysaccharide-based hydrogel drug carrier. The in-situ injection-molded thiolated polysaccharide-based hydrogel and the drug carrier thereof can be gelled at human physiological temperature, and have temperature sensitivity, injectability and water absorption.
Owner:JINAN UNIVERSITY
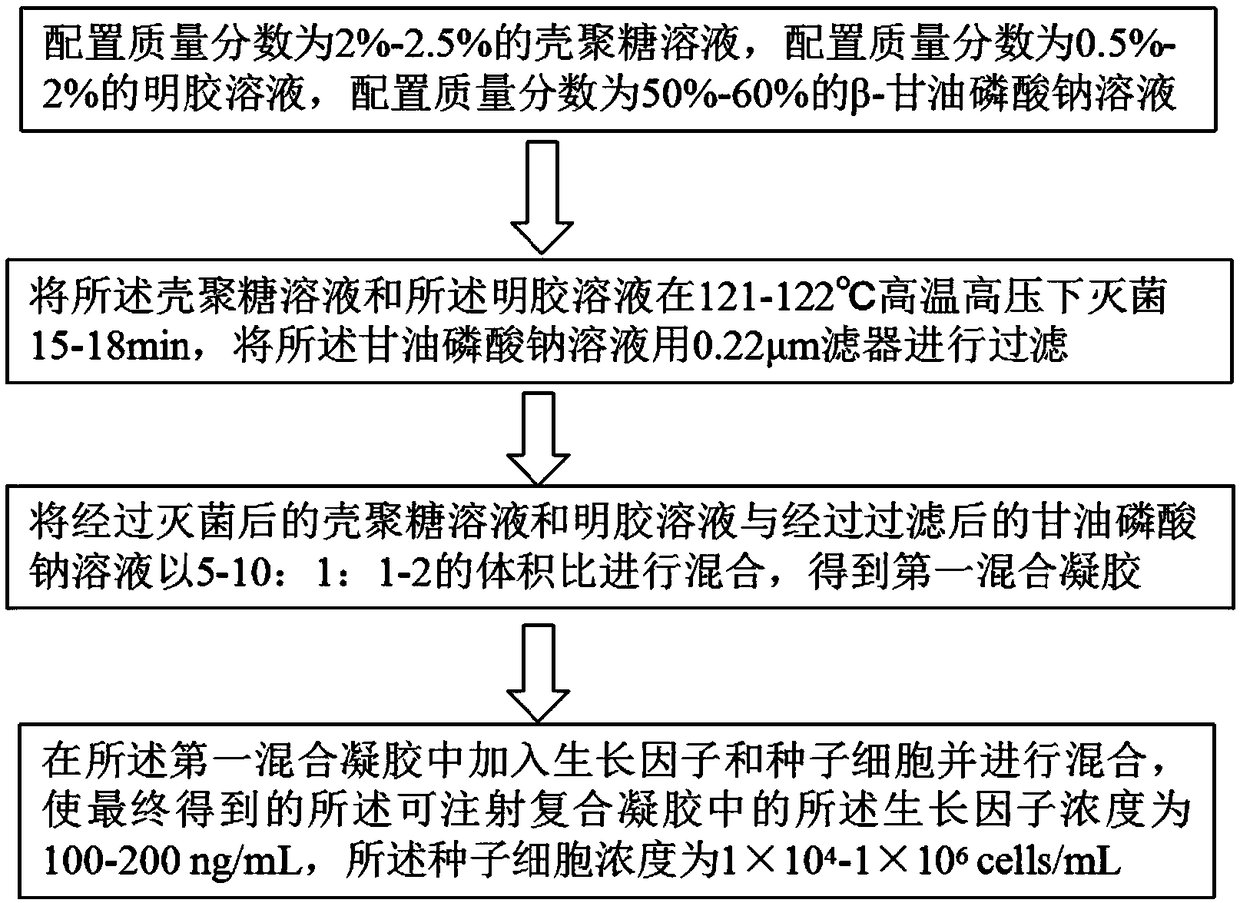
Injectable composite gel and preparation method and application thereof
InactiveCN108744057AFacilitates migration aggregationPromote differentiationPharmaceutical delivery mechanismTissue regenerationTissue repairBeta-glycerophosphate
An injectable composite gel and a preparation method and an application thereof mainly relate to the field of tissue engineering. The preparation method comprises mixing a chitosan solution, a gelatinsolution and a sodium Beta-glycerophosphate solution according to the certain ratio and sequence and adding a certain amount of growth factors and seed cells to make the prepared gel have the performance of repairing multiple kinds of tissues. The injectable composite gel prepared by the method not only has a certain factor sustained release effect but also can compound various cells to provide cell support for tissue repair, thereby better adapting to the injury part, reducing inflammation, promoting migration and aggregation of the cells and differentiation of the seed cells and promoting the formation of new blood vessels and the repair of necrotic tissues. Therefore, the injectable composite gel, the preparation method and the application have broad application prospects.
Owner:SOUTHWEST JIAOTONG UNIV
Reagent strip for detecting content of creatinine in urine and preparation method of reagent strip
InactiveCN104568931AExtended shelf lifeReduce false positive rateMaterial analysis by observing effect on chemical indicatorReagent stripCreatinine rise
The invention discloses a reagent strip for detecting the content of creatinine in urine and a preparation method of the reagent strip. The reagent strip comprises a base plate and a filter paper piece, wherein the filter paper piece is immersed with a solution A and a solution B in sequence; the solution A contains beta-glycerophosphate, sodium citrate, copper sulfate and a surfactant, and the volume is kept constant by deionized water; and the solution B contains reductive chromogen, absolute ethyl alcohol, an antioxidant, peroxide and a surfactant, and the volume is kept constant by chloroform. According to the reagent strip, the antioxidant is added to the system so that the false positive rate of the reagent strip is reduced; meanwhile, the reagent strip can be stored for a long period of time without losing efficiency; beta-glycerophosphate is used for replacing a Tris buffering solution system and the pH value of the system is controlled to be 6-7; the peroxide is introduced so that the reagent strip is very sensitive to a developing reaction of creatinine; the developing is rapid and clear; and the gradual change difference of the colors of the reagent strips in creatinine with different concentrations is obvious, and visual judgment and machine-reading judgment of operators are facilitated.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Chitosan-based plant leaf surface water evaporation retardant and preparation method thereof
InactiveCN106417262AReduce evaporationReduce evaporation rateDead plant preservationRetention timeBeta-glycerophosphate
The invention relates to a chitosan-based plant leaf surface water evaporation retardant and a preparation method thereof. A chitosan-based sol-gel system, namely, the retardant for retarding the plant leaf surface water evaporation rate, is prepared from carboxymethyl chitosan, gelatin and sodium beta-glycerophosphate through compounding. The preparation process is simple, the prepared sol system is sprayed to leaf surface, a colorless and transparent sol film layer can be formed rapidly through self-spreading, the environment temperature rises in summer, the sol film layer is changed into a thin gel layer, water can be locked by a three-dimensional network structure of gel, and leaf surface water evaporation is retarded. The chitosan-based plant leaf surface water evaporation retardant can effectively prolong the retention time of the water on the leaf surface and especially has good application feasibility in high-temperature drought regions in northwest. If the retardant is combined with a pesticide for application, the action time of the pesticide can be prolonged greatly, the action intensity of the pesticide is enhanced, and the retardant has great application value in decrement and synergism of the pesticide.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Periodontal ligament stem cells osteogenic differentiation inducing liquid and method
InactiveCN105255824AEffective osteogenic differentiationAchieve reuseSkeletal/connective tissue cellsArtificially induced pluripotent cellsDexamethasoneVitamin C
The invention relates to a periodontal ligament stem cells (PDLSCs) osteogenic differentiation inducing liquid and a method. The inducing liquid is prepared from the following components: basal culture medium, vitamin C, dexamethasone, beta-glycerophosphate sodium, FBS, IGF-1 and BMP-2. The periodontal ligament stem cell osteogenic differentiation inducing method uses the above osteogenic differentiation inducing liquid to induce. According to the multiple inducing factor osteogenic inducing liquid provided by the invention, multiple inducing factors are jointly applied in PDLSCs, so that the PDLSCs osteogenic differentiation can be effectively induced. According to the invention, the adopted raw materials are third molars required to be removed when orthodontic treatment is carried out on 12-18-year-old teenagers, and at present, the third molars are basically adopted as medical wastes to throw away; while according to the invention, the third molars are used for isolated culturing the PDLSCs to carry out osteogenic induction, so that the recycle of the medical wastes is effectively realized.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Temperature-sensitive sol
ActiveCN104887618AAchieve separationTo achieve the purpose of treatmentAerosol deliveryOintment deliveryTreatment effectBeta-glycerophosphate
The invention belongs to the technical field of medical medicine manufacturing and particularly relates to temperature-sensitive sol for treating gynecologic inflammation such as vaginitis. The temperature-sensitive sol comprises a chitosan acetic acid solution, a carboxymethyl chitosan aqueous solution and a sodium beta-glycerophosphate aqueous solution. Chitosan is dissolved into acetic acid and carboxymethyl chitosan and sodium beta-glycerophosphate are respectively dissolved into ultrapure water to obtain the three kinds of solutions, and the three kinds of solutions are evenly mixed according to set process steps to be manufactured into the temperature-sensitive sol. During use, medicine is loaded to allow the temperature-sensitive sol to be mixed with the medicine to form gel, and the gel can continuously release the medicine on the affected part for 5-7 days. The temperature-sensitive sol is scientific and reliable in principle, simple to manufacture and operate, evident in curative effect, capable of overcoming the defect that similar products can only be applied at night, and promising in application prospect.
Owner:QINGDAO UNIV
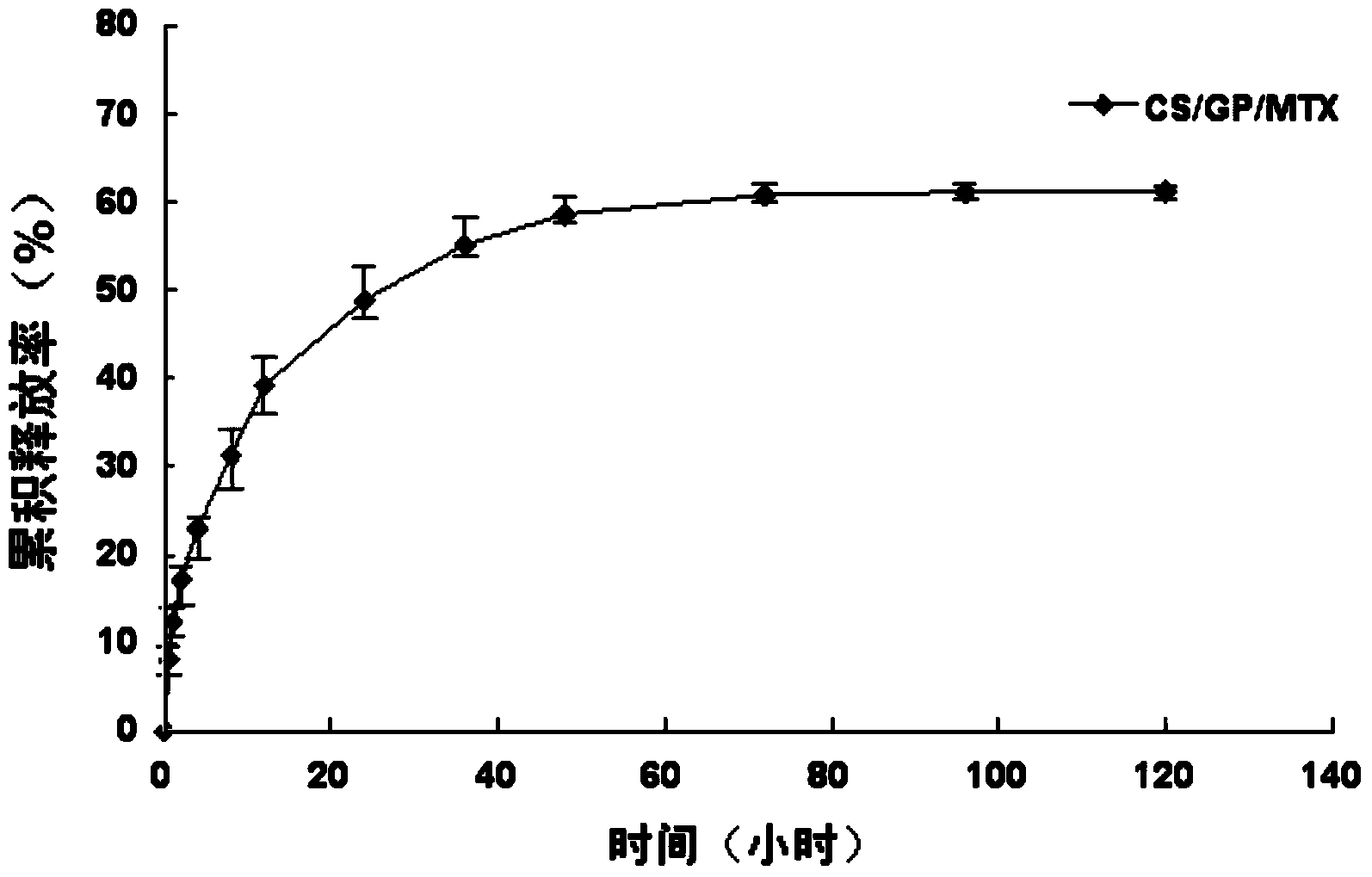
CS/GP/MAX (Methotrexate-loaded Chitosan-based Thermosensitive Hydrogel) and application thereof
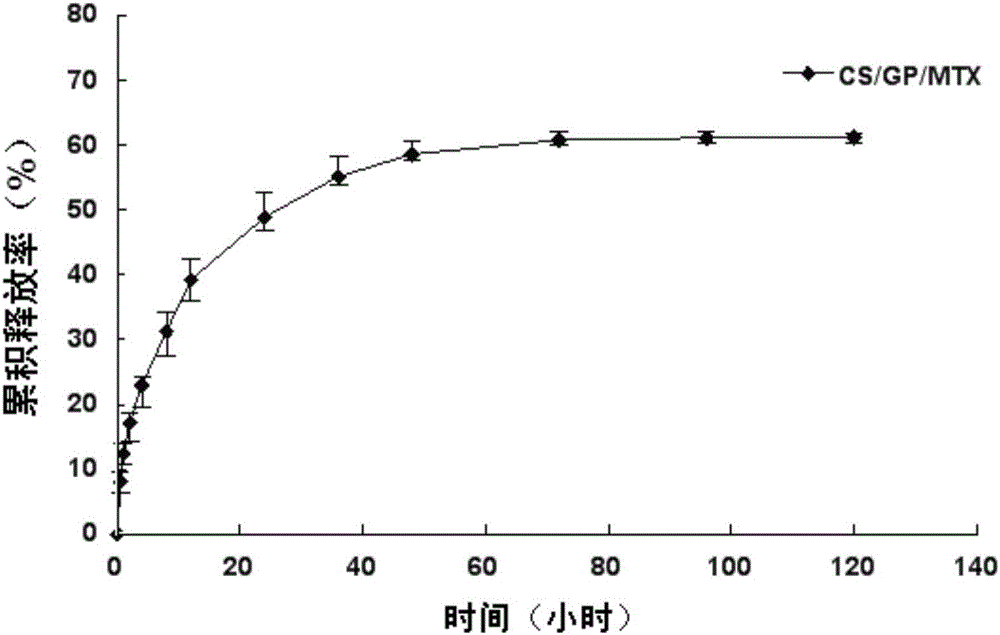
ActiveCN103735501AStable in natureQuality is easy to controlOrganic active ingredientsAntipyreticPhosphateBeta-glycerophosphate
The invention provides MTX subcutaneous sustained-release injection CS / GP / MAX (Methotrexate-loaded Chitosan-based Thermosensitive Hydrogel). Every liter of aqueous solution of the CS / GP / MAX contains 16 to 22 grams of chitosan, 0.05 to 0.1 mole of acetic acid, 70 to 150 grams of beta-glycerophosphate, 0.1 to 0.12 mole of methotrexate, 0.1 to 0.12 mole of a solubilizer and 5 to 50 grams of an isoosmotic adjusting agent and pH modifier. The CS / GP / MAX has stable properties and controllable quality, and the external properties, tgel (the time of gelation), eta, pH value, content and preliminary stability of the CS / GP / MAX meet requirements on thermosensitive hydrogel; 70 percent of drugs of the CS / GP / MAX in a PBS (Phosphate Buffer Solution) are released within 5 days under the condition of 37 DEG C; compared with those of MTX injection, the Cmax (Maximum Plasma Drug Concentration) is reduced by 82 percent, the AUC0-t (area under the plasma concentration time curve from time 0 to t hours) is enlarged by 2.37 times, and the t0.02muM (the length of time the MTX concentration-time curve remained above 0.02 muM in one week) is prolonged by 12.93 times after the CS / GP / MAX is subcutaneously injected to the back of a rabbit; by the CS / GP / MAX, adverse effects can be reduced, and curative effects can be improved.
Owner:CHANGSHA JINGYI PHARM TECH CO LTD
General medium for determination of lactobacillus resistance to drugs and use thereof
ActiveCN104946723AReduce labor intensityReduce testing costsMicrobiological testing/measurementDisodium glycerophosphateBeta-glycerophosphate
The invention discloses a general medium for determination of lactobacillus resistance to drugs and a use thereof. Each 1000mL of the general medium comprises 7-15g of peptone, 3-7g of beef extract powder, 2-4g of yeast powder, 15-25g of glucose, 0.8-1.2g of scorbic palmitate, 2.6mg of vitamin B6, 0.5-1g of cysteine hydrochloride, 1.0-2.0g of poloxamer, 0.1-0.3g of magnesium sulfate, 0.05g of MnSO4.4H2O, 15-25g of disodium beta-glycerophosphate, 15-20g of agar and the balance water and has pH of 6.2+ / -0.2. The general medium can be used for simultaneous growth of three different strains in lactobacillus, has components which do not influence various test antibiotics, greatly reduces a detection cost, reduces processes and reduces labor intensity of workers.
Owner:BEIJING JUNLIKANG BIOTECHNOLOGY CO LTD
Novel hydrochloric acid topotecan intratumor injection preparation composition and preparation method thereof
InactiveCN103479568AAvoid open loopReduce biological activityOrganic active ingredientsAerosol deliverySide effectFreeze-drying
The invention relates to a novel hydrochloric acid topotecan intratumor injection preparation composition and a preparation method thereof, belonging to the field of medicament preparations. According to the composition, a lipidosome technique and an in-situ gel technique are combined and used, the main medicine component, namely, hydrochloric acid topotecan, is carried into a lipid bilayer film through an active medicine carrying method; and the temperature sensitive gel of chitosan / sodium beta-glycerophosphate is used as a gel material which can be kept be liquid at a low temperature, and is converted into solid when the temperature is risen to the body temperature. The invention discloses the preparation method of the composition. The method comprises the following steps of preparing the hydrochloric acid topotecan lipidosome by using an ammonium sulfate gradient method, selecting a freeze-drying protecting agent, determining the concentration of the chitosan and the sodium beta-glycerophosphate, and preparing the composition. Due to the composition and the preparation method, on the basis that active components are protected and the stability of the preparation is improved, the release of the medicine is controlled, and the biological utilization degree of the main medicine is improved; due to adoption of an intratumor injection mode, the side effect of the main medicine component is reduced, and the compliance of a patient is improved; and the composition is easy to fill, and the industrial production is facilitated.
Owner:CHINA PHARM UNIV
Temperature-sensitive controlled-release pharmaceutical composition of taxane drugs
ActiveCN104784109AEasy to makeHigh drug loadingOrganic active ingredientsAerosol deliveryControlled releaseBeta-glycerophosphate
The invention relates to a temperature-sensitive controlled-release pharmaceutical composition of taxane drugs. Specifically speaking, the invention relates to the pharmaceutical composition which comprises taxane drugs, chitosan, sodium beta-glycerophosphate, ethanol and water, and further relates to a preparation method for the pharmaceutical composition. The pharmaceutical composition can be used for preparation of anticancer drugs. In particular, the anticancer drugs are applied to treatment of malignant tumors or prevention of recurrence after operation through a stereotaxical injection approach and can be cooperatively used with radiotherapy and traditional Chinese medicine.
Owner:王子厚
Anti-uterine adhesion material
The invention discloses an intrauterine adhesion prevention material, and belongs to the field of medical materials. The intrauterine adhesion prevention material is characterized in that the intrauterine adhesion prevention material is a temperature-sensitive gel; the phase transition temperature of the temperature-sensitive gel is 25-40 degrees; the temperature-sensitive gel consists of a gel main body, a tissue affinity agent, a humectant, a gelling temperature regulation agent and pure water; the gel main body is one or several of poloxamer 407, a polylactide acid / glycolic acid / polyethylene glycol copolymer, poly(N-isopropylacrylamide), chitosan, sodium beta-glycerophosphate, methoxy polyethylene glycol-(sebacic acid-D,L-lactic acid)polyester anhydride-methoxy polyethylene glycol triblock copolymer, cellulose, a cellulose derivative, and polyethylene glycol / polycaprolactone block copolymer; the tissue affinity agent is hyaluronic acid or sodium hyaluronate; the humectant is glycerol or sodium alginate; and the gelling temperature regulation agent is poloxamer 188 or polyethylene glycol. The intrauterine adhesion prevention material provided for people is good in liquidity and can be coated uniformly, and the surface of a wound can be covered quickly and completely.
Owner:广州市弘健生物医用制品科技有限公司
Method for improving osteogenic differentiation efficiency of human amniotic mesenchymal stem cells and application thereof
InactiveCN106497871AAvoid High Immunogenicity IssuesImprove the efficiency of osteogenic differentiationCulture processSkeletal/connective tissue cellsBeta-glycerophosphateSodium glycerophosphate
The invention relates to a method for improving osteogenic differentiation efficiency of human amniotic mesenchymal stem cells. The method adds an induction composition in the human amniotic mesenchymal stem cells, wherein the composition comprises 0.1-2g / L of hyaluronic acid, 0-200mg / L of prostaglandin receptor-2 selective agonist, 1-100nmol / L of dexamethasone, 0-10nmol / L of beta-glycerophosphate and 1-100mg / L of ascorbic acid. Compared with a conventional inducing method, the osteogenic differentiation efficiency of the human amniotic mesenchymal stem cells can be significantly improved, expression and activity of alkaline phosphatase are promoted, and expression of osteogenesis-related genes of RunX2, Osx, BSP, Ocn, ALP, Col1a1 and the like and formation of mineralized calcium nodules are enhanced. The method for improving the osteogenic differentiation efficiency of the human amniotic mesenchymal stem cells can be applied in osteogenic differentiation of the human mesenchymal stem cells, and applied in bone diseases of bone defects, bone trauma and the like.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Reinforced degradable intravascular stent material and preparation method thereof
InactiveCN106139245ALow hemolysis rateStrong thicknessProsthesisGlycidyl methacrylateDisodium glycerophosphate
The invention discloses a reinforced degradable intravascular stent material and a preparation method thereof. The reinforced degradable intravascular stent material comprises the following components: L-polylactide, polyglycolic acid, chitosan oligosaccharide, glycidyl methacrylate, aliskiren hemifumarate, nizofenone fumarate, D-threitol, erythritol, glycodeoxycholic acid sodium, silk fibroin, L-eucine ethyl ester hydrochloride, sodium acryloyldimethyl taurate, disodium beta-glycerophosphate, itaconyl chitosan, nano zirconium dioxide, acetic acid, dichloromethane and hexafluoroisopropanol. The stent material provided by the invention reaches degradation time of 65 to 79 days, has a hemolytic rate of lower than 3.5%, is excellent in blood compatibility, and can reduce adverse effects which cause thrombus; moreover, a stent made of the reinforced degradable intravascular stent material is high in supporting force and strong in anti-fracture strain capacity, a thickness of the stent can be further reduced, and the reinforced degradable intravascular stent material is beneficial to blood circulation and application of small molecule vessels. The stent material provided by the invention is safer, more reliable and more effective to use and has an excellent application prospect.
Owner:林春梅
Biological agent for inducing dentin mineralization
InactiveCN103445969AEfficient formationInhibit cariesImpression capsDentistry preparationsBiotechnologyDexamethasone
The invention discloses a biological agent for inducing dentin mineralization. The biological agent comprises pyrrolidine dithiocarbamate. A method for preparing the biological agent in unit dose comprises the following steps: mixing 20umol / L of pyrrolidine dithiocarbamate, 1*10<-8>mol / L of dexamethasone, 10mmol / L of sodium-beta-glycerophosphate and 50ug / mL of vitamin C, and then adding 100mL / L of alpha-MEM culture solution of fetal bovine serum, thus preparing the biological agent. The biological agent can promote the biological mineralization process of hard tissues of teeth and effectively form a reparative dentinal bridge, thus inhibiting the occurrence and development processes of caries.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
A method for inducing bone marrow mesenchymal stem cells to differentiate into osteoblasts in vitro and induction medium
InactiveCN103667182BFully demonstrate the differentiation abilityConducive to survivalSkeletal/connective tissue cellsPenicillinCulture fluid
The invention provides an inducing method and inducing culture medium for differentiation of bone marrow mesenchymal stem cells into osteoblasts in vitro. The inducing culture medium is composed of 1*10<-8> mol / L of dexamethasone, 50 mu mol / L of ascorbic acid and 10 mmol / L of sodium beta-glycerophosphate; and solvent is a supernatant of a sclerite complete culture medium and comprises 10% of fetal calf serum, 100 U / mL of penicillin, 100 mg / L of streptomycin, a mixture of DMEM culture fluid and F12 culture fluid and multiple growth factors secreted by bone cells in the sclerite culture process. According to the invention, bone marrow mesenchymal stem cells of a mouse are purified by replacing the cell culture fluid through an adherent cell passage method, the obtained cells of the first generation are induced, and the supernatant of the sclerite complete culture medium cultured for 72-96 hours is used as the solvent of osteoblast differentiation inducer, thereby obviously improving the in vitro osteogenic differentiation efficiency of bone marrow mesenchymal stem cells.
Owner:HUZHOU CENT HOSPITAL
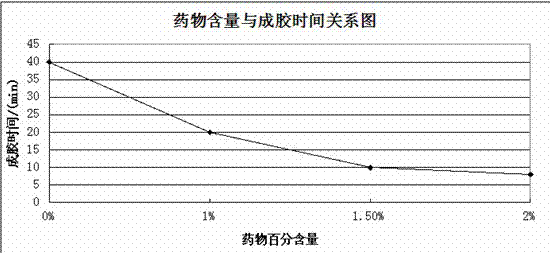
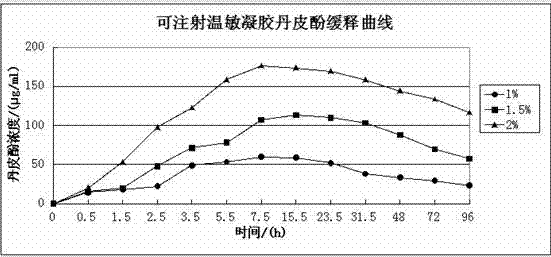
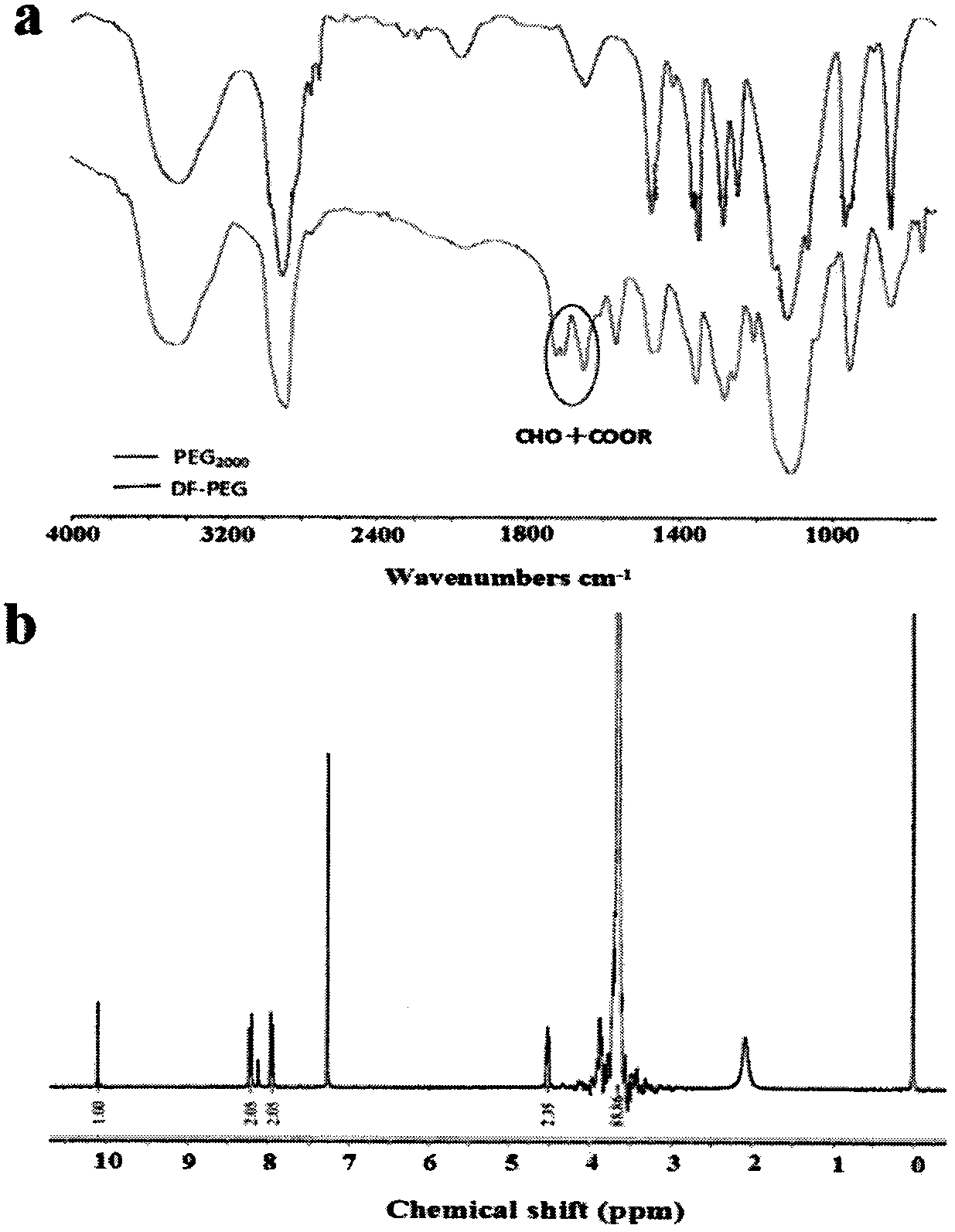
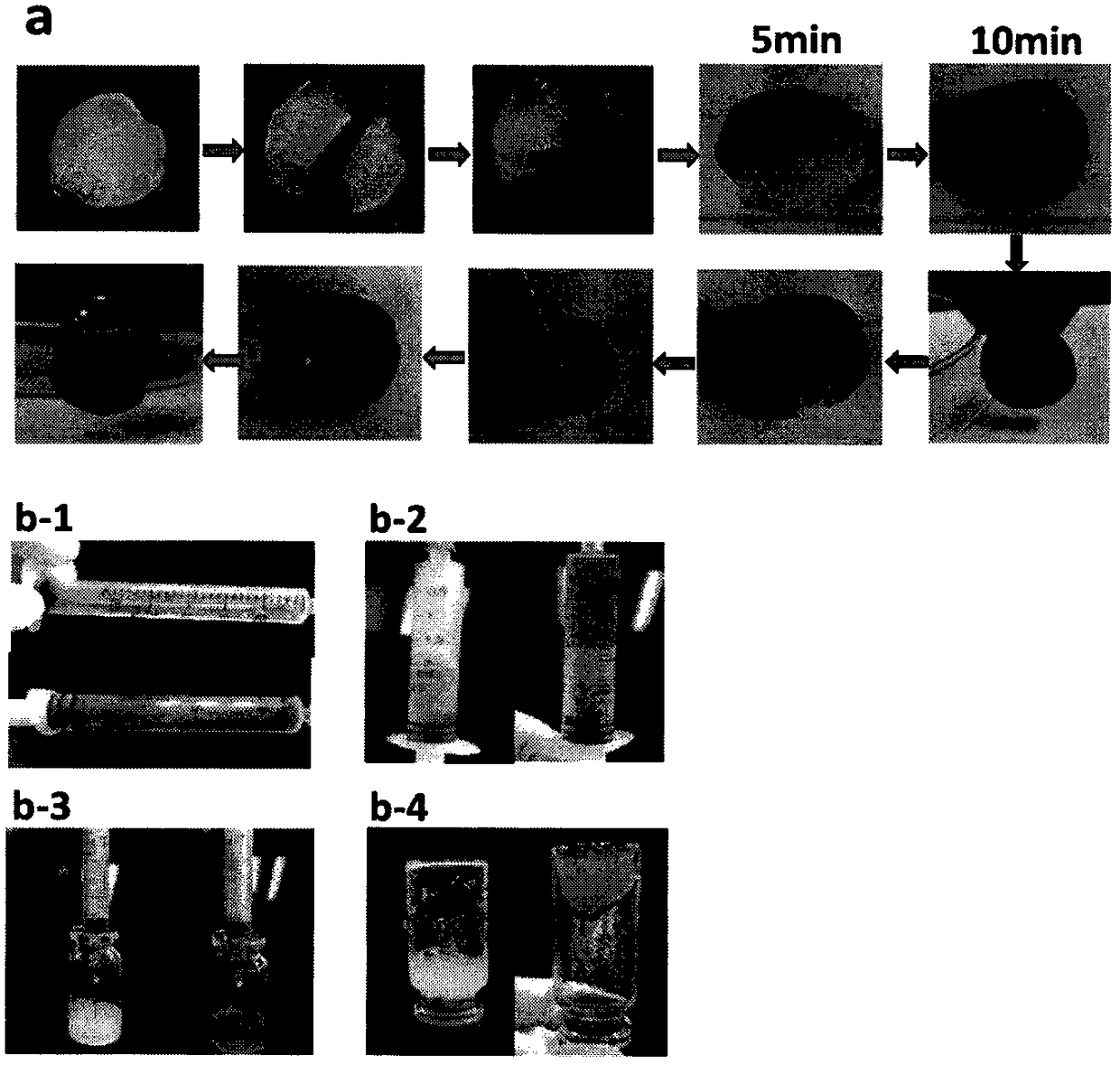
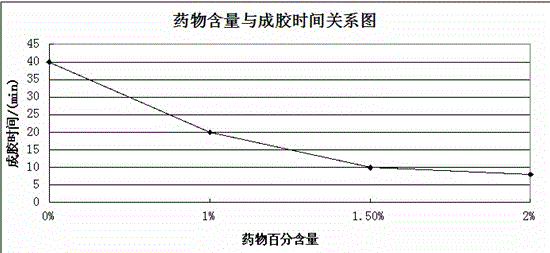
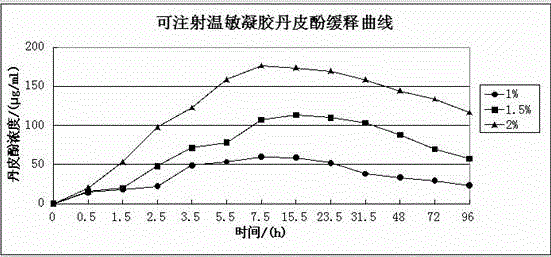
Injection type directional sustained-release paeonol thermosensitive gel and its preparation method
InactiveCN103932976AActive influenceChanging the condition of the dissolution mediumAerosol deliveryOintment deliveryWater basedBeta-glycerophosphate
The invention relates to an injection type directional sustained-release paeonol thermosensitive gel and its preparation method. The gel is obtained by dissolving paeonol in a water based solution, and solidifying, and the water based solution is prepared by using chitosan, sodium beta-glycerophosphate, a hydrochloric acid solution, a sodium hydroxide solution and deionized water through certain steps according to a certain ratio. The method concretely comprising the following steps: rapidly dissolving paeonol in the water based solution at 40DEG C to form an injection mixed solution, and injecting the mixed solution into 37DEG C environment to solidify in order to obtain the gel, and the above drug is uniformly dispersed in a gel matrix in a dissolving state in order to realize the slow drug release, so it is beneficial for realizing the minimally invasive filling of defect tissues, and directionally slowly release the paeonol drug to realize antibiosis, antiphlogosis and pain easing in order to make the drug effect more lasting.
Owner:HENAN UNIV OF SCI & TECH
Umbilical cord Wharton's jelly mesenchymal stem cell osteogenic directional differentiation method
ActiveCN110564678AImprove the efficiency of induced differentiationNo side effectsCell dissociation methodsCulture processBeta-glycerophosphateDirected differentiation
The invention provides an umbilical cord Wharton's jelly mesenchymal stem cell osteogenic directional differentiation method and relates to the fields of stem cells and regenerative medicines. The method comprises the following steps of tissue digestion, primary culture, subculture, induction culture, etc.; in the tissue digestion step, tissue digestion liquid is used for tissue digestion, whereinthe tissue digestion liquid comprises collagenase I, DNA enzyme and Tryple; and in the induction culture step, an osteoblast differentiation induction culture medium is used for the induction culture, and the osteoblast differentiation induction culture medium comprises an alpha-MEM base bottom solution, a serum substitute, dexamethasone, beta-glycerophosphate, antibiotics, cytokine IL-beta, ascorbic acid and isoflavone. The method can obviously improve induction differentiation efficiency of umbilical cord mesenchymal stem cell osteoblasts.
Owner:GUANGDONG VITALIFE BIOTECHNOLOGY CO LTD
Injectable chitosan composite hydrogel for promoting myocardial repair and preparation method thereof
The invention discloses injectable chitosan / keratin composite hydrogel capable of promoting myocardium repair and a preparation method of the hydrogel. The hydrogel consists of four solutions, namely chitosan, sodium beta-glycerophosphate, genipin and keratin according to a volume ratio of 10: (1-5): (1-3): (0.1-0.5). The preparation method comprises the following steps: 1) extracting keratin from human fairs by using peracetic acid and Tris alkaline; 2) adjusting the pH value of a water-soluble chitosan solution by using sodium beta-glycerophosphate so as to be close to a microenvironment in a human body; 3) introducing genipin as a crosslinking agent, and controlling the gelling time of a hydrogel solution by changing the use amount of the genipin so as to achieve the purpose that the mixed solution can be rapidly gelled in situ after being injected into an organism. Experiments prove that the composite hydrogel has favorable biocompatibility and has the capability of remarkably promoting the growth of myocardial cells; the hydrogel is wide in sources of materials and low in cost and has a broad application prospect in the field of tissue engineering repair.
Owner:SOUTHEAST UNIV
Bone material preserving fluid and its prepn and use
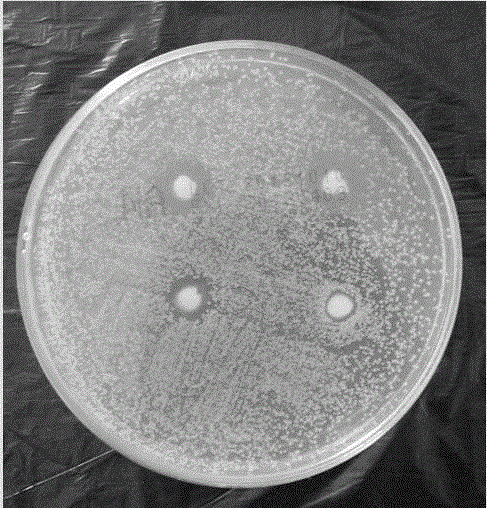
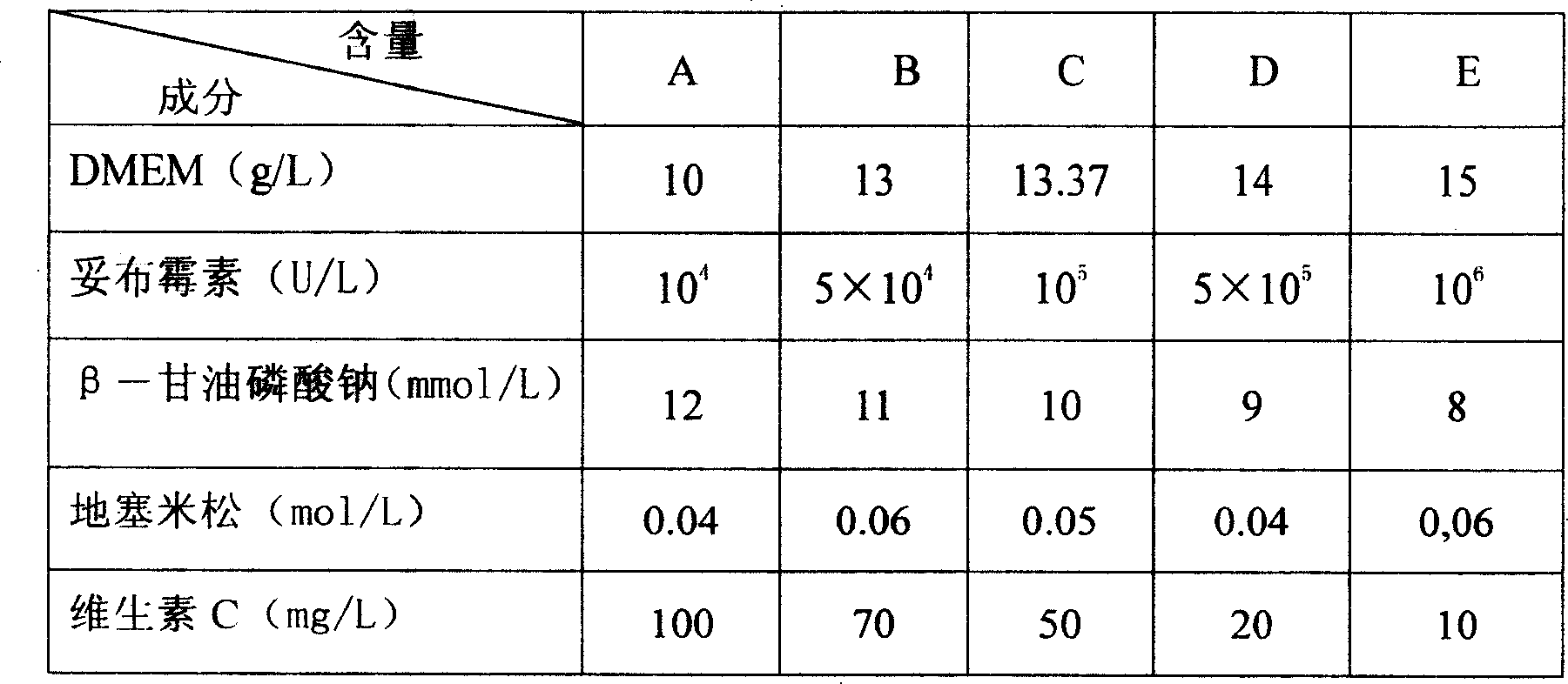
InactiveCN100429975CImprove antibacterial propertiesGood biocompatibilityDead animal preservationVitamin COsteoblast
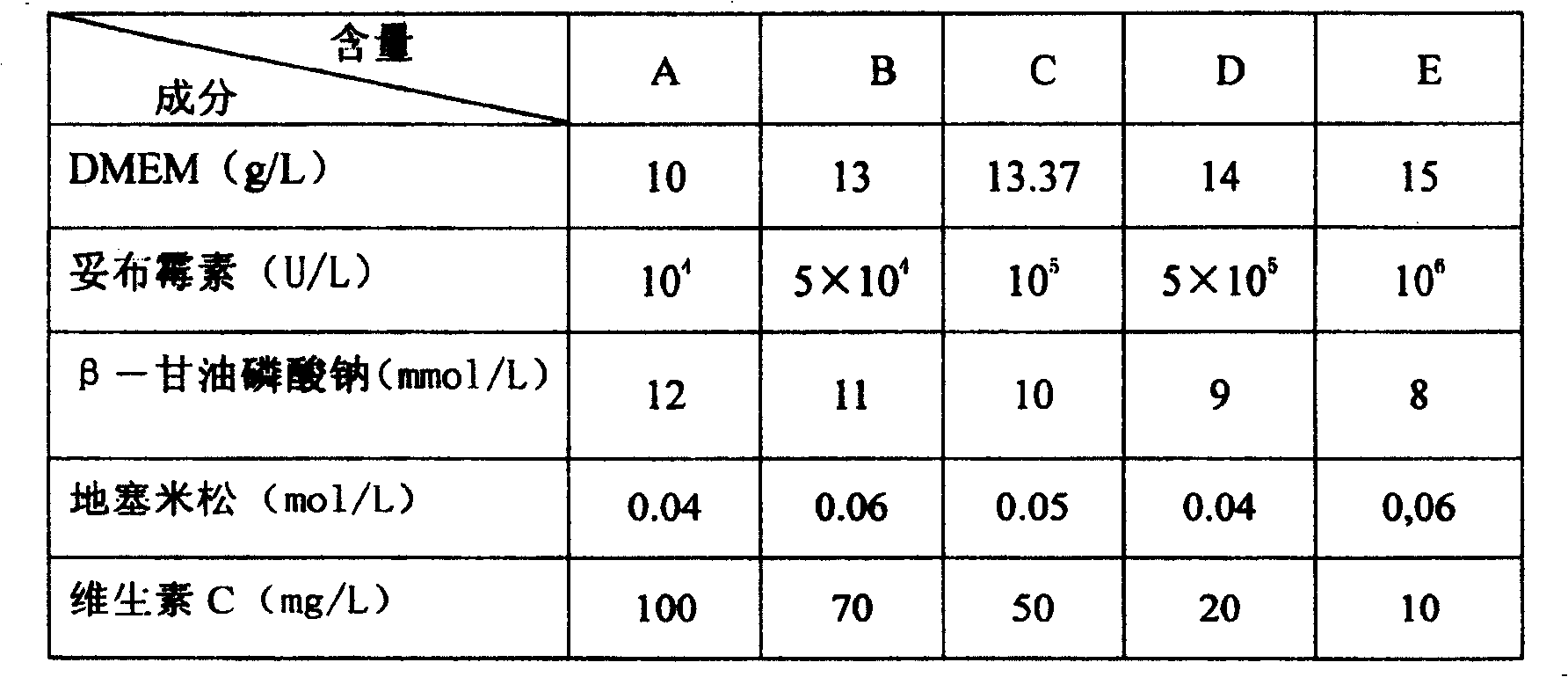
The present invention relates to one kind of bone material preserving fluid and its preparation and use. The bone material preserving fluid consists of osteoblast culture medium in 10-15g / L, sodium beta-glycerophosphate in 8-12 mmol / L, dexamethasone in 0.02- 0.08 mmol / L, vitamin C in 10-100 mg / L, tobramycin in 10<4>-10<6> U / L or tetracycline in 4-12 mg / L, and water as the solvent. The bone material preserving fluid is used in preserving bone repairing material, especially biologically derived bone material, at normal temperature. It can maintain the physical and chemical performances of bone material, enhance its antibiotic performance and cell compatibility, and raise its atopic osteoplastic effect and bone defect repairing effect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Thermosensitive slow-release pharmaceutical composition of taxane drugs
ActiveCN104784109BEasy to makeHigh drug loadingOrganic active ingredientsAerosol deliveryControl releaseBeta-glycerophosphate
The invention relates to a temperature-sensitive controlled-release pharmaceutical composition of taxane drugs. Specifically speaking, the invention relates to the pharmaceutical composition which comprises taxane drugs, chitosan, sodium beta-glycerophosphate, ethanol and water, and further relates to a preparation method for the pharmaceutical composition. The pharmaceutical composition can be used for preparation of anticancer drugs. In particular, the anticancer drugs are applied to treatment of malignant tumors or prevention of recurrence after operation through a stereotaxical injection approach and can be cooperatively used with radiotherapy and traditional Chinese medicine.
Owner:王子厚
Methotrexate chitosan thermosensitive gel and its application
ActiveCN103735501BStable in natureQuality is easy to controlOrganic active ingredientsAntipyreticPhosphateBeta-glycerophosphate
The invention provides MTX subcutaneous sustained-release injection CS / GP / MAX (Methotrexate-loaded Chitosan-based Thermosensitive Hydrogel). Every liter of aqueous solution of the CS / GP / MAX contains 16 to 22 grams of chitosan, 0.05 to 0.1 mole of acetic acid, 70 to 150 grams of beta-glycerophosphate, 0.1 to 0.12 mole of methotrexate, 0.1 to 0.12 mole of a solubilizer and 5 to 50 grams of an isoosmotic adjusting agent and pH modifier. The CS / GP / MAX has stable properties and controllable quality, and the external properties, tgel (the time of gelation), eta, pH value, content and preliminary stability of the CS / GP / MAX meet requirements on thermosensitive hydrogel; 70 percent of drugs of the CS / GP / MAX in a PBS (Phosphate Buffer Solution) are released within 5 days under the condition of 37 DEG C; compared with those of MTX injection, the Cmax (Maximum Plasma Drug Concentration) is reduced by 82 percent, the AUC0-t (area under the plasma concentration time curve from time 0 to t hours) is enlarged by 2.37 times, and the t0.02muM (the length of time the MTX concentration-time curve remained above 0.02 muM in one week) is prolonged by 12.93 times after the CS / GP / MAX is subcutaneously injected to the back of a rabbit; by the CS / GP / MAX, adverse effects can be reduced, and curative effects can be improved.
Owner:CHANGSHA JINGYI PHARM TECH CO LTD
Preparing method of pearl layer powder - chitosan porous scaffold
InactiveCN106310377AUniform pore sizeHigh biosecurityTissue regenerationProsthesisTissue defectBeta-glycerophosphate
The invention discloses a preparing method of pearl layer powder - chitosan porous scaffold, comprising the steps of (1) adding chitosan into acetic acid solution to obtain chitosan solution and standing for 24-36 h; (2) dissolving sodium Beta-glycerophosphate in distilled water to obtain the sodium Beta- glycerophosphate solution; (3) mixing the solutions from step (1) and step (2) at the volume ratio of 7:1 and stirring for 2 h under ice bath, and adjusting pH with acetic acid and sodium hydroxide solution; (4) adding nacre powder and stirring for 48 h under ice bath, pouring into mould, standing, lyophilizing and sterilizing to obtain nacre powder / chitosan porous scaffold. The compound scaffold has porous structure with uniform pore size, good biological safety and good mechanical properties, and can be applied as tissue engineering scaffold of non-bearing part in the course of repairing tissue defect.
Owner:SHAANXI YIPINDA PETROCHEM CO LTD
Novel temperature-sensitive hydrogel-like sealant and lubricant for dental implant system
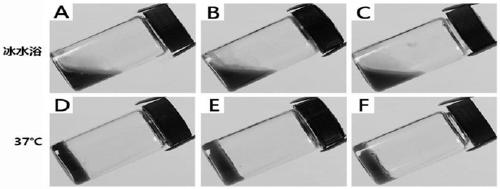
ActiveCN109010077AImprove stabilityPlay a lubricating roleImpression capsDentistry preparationsLiquid temperatureIce water
The invention belongs to the field of biomedical technology, and in particular relates to a temperature-sensitive hydrogel-like sealant and lubricant for a dental implant system. The preparation method of the temperature-sensitive hydrogel-like sealant and lubricant is as follows: dissolving chitosan powder in glacial acetic acid to obtain a chitosan solution, and sterilizing the chitosan solutionat high temperature and pressure; dissolving sodium beta-glycerophosphate and povidone iodine in filtered and sterilized tri-distilled water separately to obtain a sodium beta-glycerophosphate solution and a povidone iodine solution; mixing the chitosan solution and the povidone iodine solution, and stirring evenly to obtain a solution A; putting the solution A and the sodium beta-glycerophosphate solution in an ice-water bath simultaneously, then slowly dripping the sodium beta-glycerophosphate solution into the solution A under stirring conditions, fully stirring uniformly the solution in the ice-water bath to obtain a liquid temperature-sensitive hydrogel. The preparation method is simple, low in production cost, and convenient for popularization and use.
Owner:WUHAN UNIV
Ferrite/bacille calmette-guerin vaccine/temperature sensing gel integration material and preparation thereof
InactiveCN100560136CFunctionalImprove efficacyBacterial antigen ingredientsInorganic non-active ingredientsWater bathsFreeze-drying
The invention discloses a ferrite / BCG / temperature-sensitive gel integrated material, which comprises 5%-15% of BCG, 5%-15% of ferric oxide, 70%-90% of chitosan / Sodium β-glycerophosphate xerogel. The preparation method of the integrated material is to prepare nano ferric oxide by co-precipitation method; dissolve chitosan in dilute hydrochloric acid, add quantitatively prepared ferric oxide and BCG, disperse evenly, and then add β-sodium glycerophosphate dropwise The solution is heated in a water bath to convert the mixed solution into a gel; the obtained gel is freeze-dried or vacuum-dried to obtain a chitosan xerogel; the xerogel is ground and sieved to obtain a corresponding integrated material. The integrated material prepared by the present invention has the function of targeting wall attachment and sustained release. The integrated material powder can be attached to the inner wall of the bladder under the action of an external magnetic field so as not to be excreted with urine, prolonging the time it stays in the bladder and strengthening the BCG. efficacy.
Owner:SHANDONG UNIV
A temperature-sensitive self-healing hydrogel loaded with doxorubicin hydrochloride and its preparation method
InactiveCN105520906BReduce releaseAvoid sudden releaseOrganic active ingredientsAerosol deliverySelf-healingSide effect
The invention relates to a doxorubicin hydrochloride loaded temperature-sensitive self-healing hydrogel and a preparation method thereof. The doxorubicin hydrochloride loaded temperature-sensitive self-healing hydrogel comprises chitosan, sodium beta-glycerophosphate, and polyethylene glycol with two ends respectively carrying a benzaldehyde group. The preparation method comprises the following steps: forming an imide bond with a self-healing performance through using a reaction of an amino group in the structure of chitosan and the benzaldehyde groups at two ends of the polyethylene glycol, combining the imide bond with chitosan / sodium beta-glycerophosphate temperature-sensitive gel, and loading doxorubicin hydrochloride into the obtained material through physical clathration to prepare the novel temperature-sensitive self-healing hydrogel administration system. The administration system is a liquid at room temperature, and rapidly gelates at a human body temperature after being injected to a tumor position in order to form a medicine reservoir ; and compared with traditional in situ gels, the temperature-sensitive self-healing hydrogel has the advantages of strong mechanical strength, realization of rapid self restoration under the action of outside force or tissue damages to avoid burst release of a medicine, further delay of release of the medicine and prolongation of the detention time of the medicine in the tumor position, medicine effect enhancement and reduction of toxic and side effects.
Owner:CHINA PHARM UNIV
An injection-type directional slow-release paeonol temperature-sensitive gel and its preparation method
InactiveCN103932976BActive influenceChanging the condition of the dissolution mediumAerosol deliveryOintment deliveryWater basedBeta-glycerophosphate
The invention relates to an injection type directional sustained-release paeonol thermosensitive gel and its preparation method. The gel is obtained by dissolving paeonol in a water based solution, and solidifying, and the water based solution is prepared by using chitosan, sodium beta-glycerophosphate, a hydrochloric acid solution, a sodium hydroxide solution and deionized water through certain steps according to a certain ratio. The method concretely comprising the following steps: rapidly dissolving paeonol in the water based solution at 40DEG C to form an injection mixed solution, and injecting the mixed solution into 37DEG C environment to solidify in order to obtain the gel, and the above drug is uniformly dispersed in a gel matrix in a dissolving state in order to realize the slow drug release, so it is beneficial for realizing the minimally invasive filling of defect tissues, and directionally slowly release the paeonol drug to realize antibiosis, antiphlogosis and pain easing in order to make the drug effect more lasting.
Owner:HENAN UNIV OF SCI & TECH
Bone material preserving fluid and its prepn and use
InactiveCN101019528AImprove antibacterial propertiesGood biocompatibilityDead animal preservationVitamin COsteoblast
The present invention relates to one kind of bone material preserving fluid and its preparation and use. The bone material preserving fluid consists of osteoblast culture medium in 10-15g / L, sodium beta-glycerophosphate in 8-12 mmol / L, dexamethasone in 0.02- 0.08 mmol / L, vitamin C in 10-100 mg / L, tobramycin in 10<4>-10<6> U / L or tetracycline in 4-12 mg / L, and water as the solvent. The bone material preserving fluid is used in preserving bone repairing material, especially biologically derived bone material, at normal temperature. It can maintain the physical and chemical performances of bone material, enhance its antibiotic performance and cell compatibility, and raise its atopic osteoplastic effect and bone defect repairing effect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com