Temperature-sensitive controlled-release pharmaceutical composition of taxane drugs
A technology of taxanes and compositions, applied in the field of medicine, can solve the problems of patient suffering, dose-dependent immunosuppression, hypersensitivity reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1: preparation pharmaceutical composition of the present invention
[0089] formula
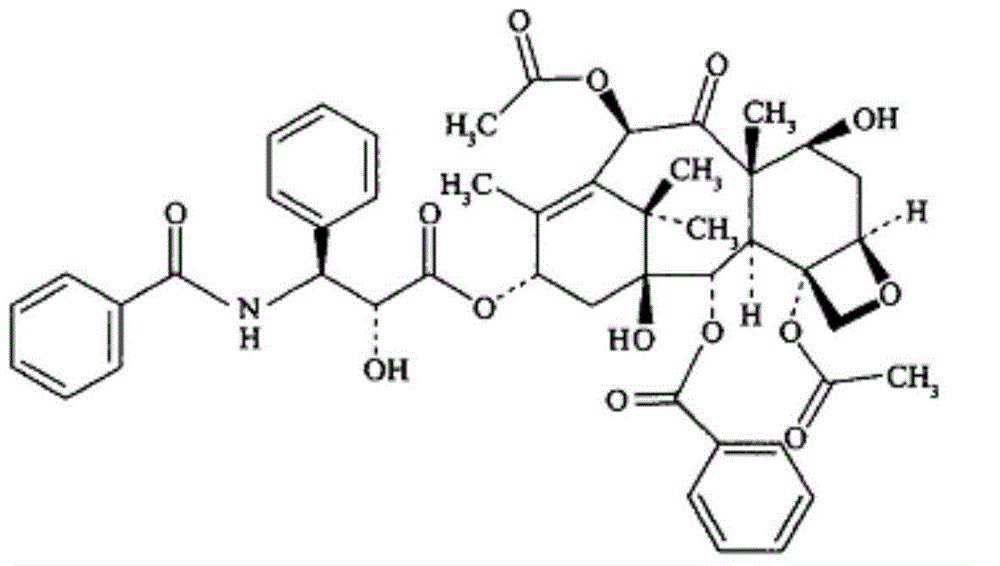
[0090] paclitaxel
0.2g
2g
Sodium β-glycerophosphate
10g
2.5g
Cit
1 / 100
water
Appropriate amount, add to 100g
Final solution pH
7.0
[0091] Preparation: under the conditions of preparing sterile preparations, prepare according to the following steps:
[0092] a) dissolving chitosan with an aqueous solution (0.1M acetic acid) of an acidic reagent is made into a 2.5% chitosan solution;
[0093] b) dissolving taxanes in ethanol, then slowly adding it to the above-mentioned chitosan solution, autoclaving, cooling in an ice-water bath for at least 30 minutes, and setting aside;
[0094] c) Dissolve β-glycerophosphate sodium in an appropriate amount of water for injection to make a solution with a concentration of 60%, pass through a 0.22 μm filter membrane to sterilize, coo...
Embodiment 2
[0097] Embodiment 2: preparation pharmaceutical composition of the present invention
[0098] formula:
[0099] paclitaxel
0.05g
3g
Sodium β-glycerophosphate
5g
ethanol
5g
Cit
0.5 / 100
water
Appropriate amount, add to 100g
Final solution pH
6.5
[0100] Preparation: Carry out with reference to the method of Example 1, use 0.1M hydrochloric acid to dissolve chitosan in step a).
[0101] The results of fluidity measurement: gelation time (t1, 37°C) = 7min, decoagulation time (t2, 20°C) = 56min.
Embodiment 3
[0102] Embodiment 3: preparation pharmaceutical composition of the present invention
[0103] formula:
[0104] paclitaxel
0.5g
1g
Sodium β-glycerophosphate
15g
ethanol
1g
Cit
2 / 100
water
Appropriate amount, add to 100g
Final solution pH
7.5
[0105] Preparation: Carry out with reference to the method of Example 1.
[0106] The results of fluidity measurement: gelling time (t1, 37°C) = 57min, defrosting time (t2, 20°C) = 12min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com