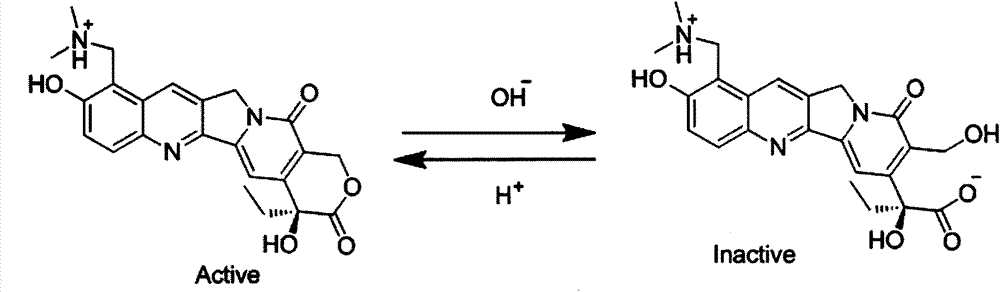
Novel hydrochloric acid topotecan intratumor injection preparation composition and preparation method thereof
A technology for intratumoral injection of topotecan hydrochloride, applied in the field of intratumoral injection preparations and its preparation, new topotecan hydrochloride intratumoral injection preparation composition and its preparation fields, to achieve improved stability, reduced side effects, and easy infusion The effect of pretending
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0032] Weigh 0.8g of soybean lecithin and 0.08g of cholesterol, add 10mL of dichloromethane to dissolve. Dichloromethane was removed by rotary evaporation at 35°C, and dried in vacuum for 2 hours to remove residual organic solvent. Add 20mL of 0.3mol / L ammonium sulfate solution and hydrate at 35°C for 30min. High-pressure homogeneous treatment, granulation through 0.22μm microporous membrane. Add 80mg of topotecan hydrochloride powder, stir to dissolve, incubate at 65°C for 20min, pass through a 0.22μm microporous membrane to obtain the finished liposome. 5% lactose and 5% sucrose were selected as freeze-drying protective agents, and the liposomes were freeze-dried. The liposome encapsulation efficiency was 86.9%.
[0033] Add chitosan (0.05g) to acetic acid solution (0.1M), stir evenly, add 3 mg of the prepared topotecan hydrochloride liposome lyophilized powder, stir evenly, place in ice water bath and continue stirring for 15min, and β-glycerophosphate Sodium (48%, 0.8 ...
specific Embodiment 2
[0034] Weigh 0.8g of soybean lecithin and 0.18g of cholesterol, add 10mL of dichloromethane to dissolve. Dichloromethane was removed by rotary evaporation at 35°C, and dried in vacuum for 2 hours to remove residual organic solvent. Add 20mL of 0.3mol / L ammonium sulfate solution and hydrate at 35°C for 30min. High-pressure homogenization treatment, passing through a 0.22 μm microporous membrane for sizing. Add 80mg of topotecan hydrochloride powder, stir to dissolve, incubate at 65°C for 20min, pass through a 0.22μm microporous membrane to obtain the finished liposome. 5% lactose and 5% sucrose were selected as freeze-drying protective agents, and the liposomes were freeze-dried. The liposome encapsulation efficiency was 90.6%.
[0035] Add chitosan (0.05g) to acetic acid solution (0.1M), stir evenly, add 3 mg of the prepared topotecan hydrochloride liposome lyophilized powder, stir evenly, place in ice water bath and continue stirring for 15min, and β-glycerophosphate Sodi...
specific Embodiment 3
[0036] Weigh 0.9 g of soybean lecithin and 0.225 g of cholesterol, add 10 mL of dichloromethane to dissolve. Dichloromethane was removed by rotary evaporation at 35°C, and dried in vacuum for 2 hours to remove residual organic solvent. Add 20mL of 0.25mol / L ammonium sulfate solution and hydrate at 35°C for 30min. High-pressure homogeneous treatment, granulation through 0.22μm microporous membrane. Add 80mg of topotecan hydrochloride powder, stir to dissolve, incubate at 65°C for 20min, pass through a 0.22μm microporous membrane to obtain the finished liposome. 5% lactose and 5% sucrose were selected as freeze-drying protective agents, and the liposomes were freeze-dried. The liposome encapsulation efficiency was 93.6%.
[0037] Add chitosan (0.05g) to acetic acid solution (0.1M), stir evenly, add 3 mg of the prepared topotecan hydrochloride liposome lyophilized powder, stir evenly, place in ice water bath and continue stirring for 15min, and β-glycerophosphate Sodium (48%,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com