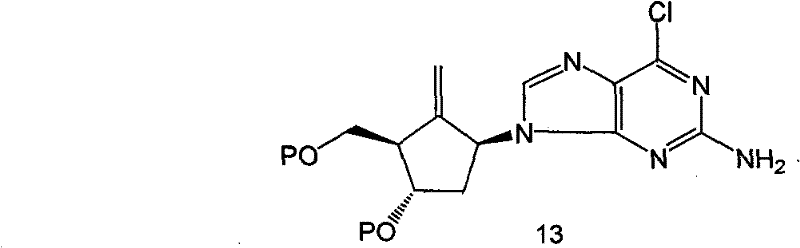
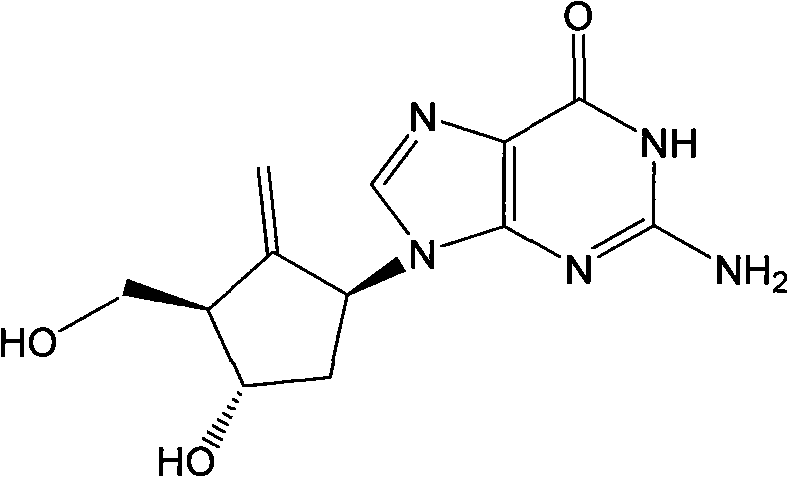
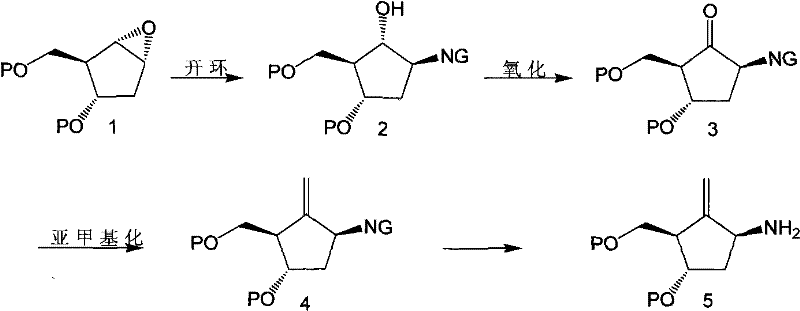
Intermediates of Entecavir and preparation thereof
A technology of compounds and structural formulas, applied in the fields of compounds, organic chemistry, chemical instruments and methods of elements of group 4/14 of the periodic table, can solve problems such as difficult separation and purification of ring-opening reaction products, complex processes, and complicated separations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: [1s-(1α, 2β, 3α, 5β)]-5-(phthalimido)-3-(benzyloxy)-2-[(benzyloxy)methyl Preparation of cyclopentanol (intermediate 2')
[0066] In a 5L three-necked flask, add 145g (0.98mol) phthalimide, 3.77g LiH and 765ml anhydrous DMF, and stir for 10min. After heating to 60°C and stirring for 15 min, the turbid liquid became clear. 152g (0.49mol) [1s-(1α, 2α, 3β, 5α)]-3-(benzyloxy)-2-[(benzyloxy)methyl] dissolved in 1.87L anhydrous DMF was slowly added dropwise. -6-oxabicyclo[3.1.0]hexane (intermediate 1'), stirred at 60°C for 15 min. It was heated to 125°C and reacted for 2h. TLC (B:positive=1:3) showed that the raw materials disappeared, and the reaction was terminated with 28ml of glacial acetic acid. Stir for 10 minutes. 2.5L of saturated brine was added, extracted with ethyl acetate 3×1.2L, the organic phases were combined and washed once with saturated brine, dried over anhydrous sodium sulfate, and the solvent was recovered. The remaining oil was separated o...
Embodiment 2
[0070] Example 2: [1s-(1α, 2β, 3α, 5β)]-5-[phthalimido]-3-(benzyloxy)-2-[(benzyloxy)methyl Preparation of yl]cyclopentanone (intermediate 3')
[0071] In a 3L three-necked flask, add 203g of Dess-Martin reagent, add 1.4L anhydrous CH 2 Cl 2 Stir. 137.7g of intermediate 2' was mixed with 890ml of anhydrous CH 2 Cl 2 Dissolved, added dropwise to the suspension in the previous step, after 20 min, TLC (B: positive = 1:3) showed that the raw materials disappeared, and the reaction was stopped. first with NaHSO 3 Washed with saturated aqueous solution 3 times, then washed with NaHCO 3 Washed with saturated aqueous solution 3 times, and finally washed with saturated brine 3 times, the organic layer was dehydrated and drained to obtain 196 g of a yellow oily compound.
Embodiment 3
[0072] Example 3: 1s-(1α,3α,4β)-5-phthalimido-2-methylene-4-(benzyloxy)-3-[(benzyloxy)methyl Preparation of cyclopentane (intermediate 4')
[0073] In a 5L three-necked flask, add 1.46L of Nysted Reagent (Wt=20%) and 800ml of anhydrous THF, stir, N 2 Protect and cool to -78°C. 196g of intermediate 3' was added to an appropriate amount of CH 2 Cl 2 Dissolved and added dropwise to the reaction. Take TiCl 4 / CH 2 Cl 2 (1:9) 393ml was slowly added dropwise to the reaction, maintaining the temperature at -60°C to -78°C. After the dropwise addition, the mixture was maintained at -78°C for 15 min. The temperature was slowly raised to room temperature, and stirring was continued for 1-3 h. TLC (B:positive=1:4) showed that the raw materials disappeared, and the reaction solution was purple-black. The reaction solution was poured into 2.3L saturated NaHCO 3 , stir well, and white turbidity will appear at this time. Extract with ethyl acetate three times, back-extract once wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com