Preparation method for N-methylisatoic anhydride rapidly-labeled polysaccharide
A technology of methyl isatonic anhydride and polysaccharide, which is applied in the field of fluorescent labeling, can solve problems such as adverse reactions and inconvenient polysaccharides, and achieve the effects of low price, short reaction time and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
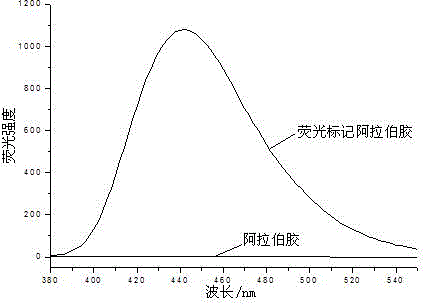
[0021] (1) Sample treatment: Dissolve 5-10 mL of 0.1 mol / L, pH 8.0 borate buffer solution and 50% w / v dimethyl sulfoxide solution to prepare a 40-80 mg / mL gum arabic solution.
[0022] (2) Preparation of fluorescent labeling stock solution: N-methylisatoic anhydride was dissolved in dimethyl sulfoxide to prepare a 20 mg / mL solution.
[0023] (3) Add the prepared fluorescent labeling stock solution to the gum arabic solution according to the mass ratio of the fluorescent label N-methyl isatoic anhydride to gum arabic at a ratio of 1:50 and incubate at 35°C for 15 minutes.
[0024] (4) Separation of fluorescently labeled polysaccharides: N-methylisatoic anhydride-labeled gum arabic was separated by organic solvent precipitation, absolute ethanol was added to the labeled polysaccharide solution, and polysaccharides were separated by alcohol precipitation. Fluorescence-labeled polysaccharide solution: volume ratio of absolute ethanol 1:4, centrifuge the alcohol-precipitated polysa...
Embodiment 2
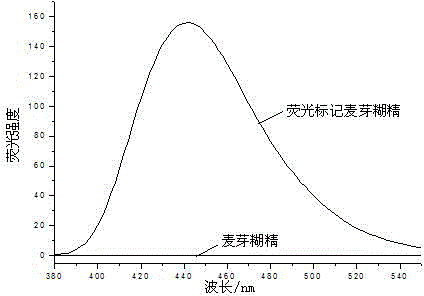
[0027] (1) Sample treatment: Dissolve 5-10 mL of 0.1 mol / L, pH 8.0 borate buffer solution and 50% w / v dimethyl sulfoxide solution to prepare a 40-80 mg / mL maltodextrin solution.
[0028] (2) Preparation of fluorescent labeling stock solution: N-methylisatoic anhydride was dissolved in dimethyl sulfoxide to prepare a 20 mg / mL solution.
[0029] (3) Add the prepared fluorescent marker stock solution to the maltodextrin solution at a mass ratio of 1:55 of the fluorescent marker N-methylisatoic anhydride to maltodextrin and incubate at 35°C for 18 minutes.
[0030] (4) Separation of fluorescently labeled polysaccharides: N-methylisatoic anhydride-labeled gum arabic was separated by organic solvent precipitation, absolute ethanol was added to the labeled polysaccharide solution, and polysaccharides were separated by alcohol precipitation. Fluorescence-labeled polysaccharide solution: volume ratio of absolute ethanol 1:6, centrifuge the alcohol-precipitated polysaccharide at 6000×g ...
Embodiment 3
[0033] (1) Sample treatment: Dissolve 5-10 mL of 0.1 mol / L, pH 8.0 borate buffer solution and 50% w / v dimethyl sulfoxide solution to prepare a 40-80 mg / mL maltodextrin solution.
[0034] (2) Preparation of fluorescent labeling stock solution: N-methylisatoic anhydride was dissolved in dimethyl sulfoxide to prepare a 20 mg / mL solution.
[0035] (3) Add the prepared fluorescent labeling stock solution to the maltodextrin solution according to the mass ratio of fluorescent label N-methylisatoic anhydride to maltodextrin of 1:60 and incubate at 35°C for 20 minutes.
[0036] (4) Separation of fluorescently labeled polysaccharides: N-methylisatoic anhydride-labeled gum arabic was separated by organic solvent precipitation, absolute ethanol was added to the labeled polysaccharide solution, and polysaccharides were separated by alcohol precipitation. Fluorescence-labeled polysaccharide solution: volume ratio of absolute ethanol 1:8, alcohol-precipitated polysaccharides were centrifuge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com