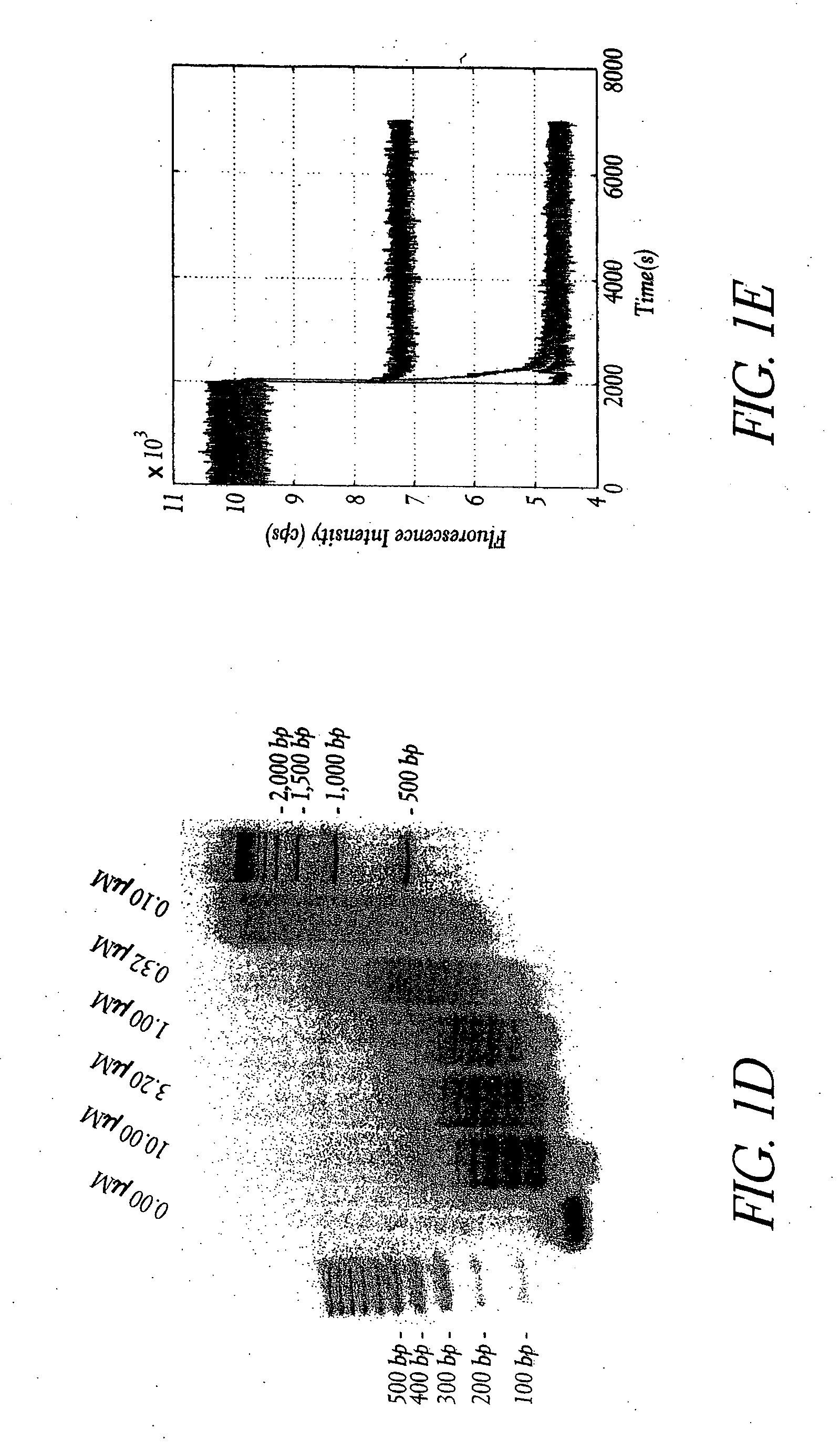
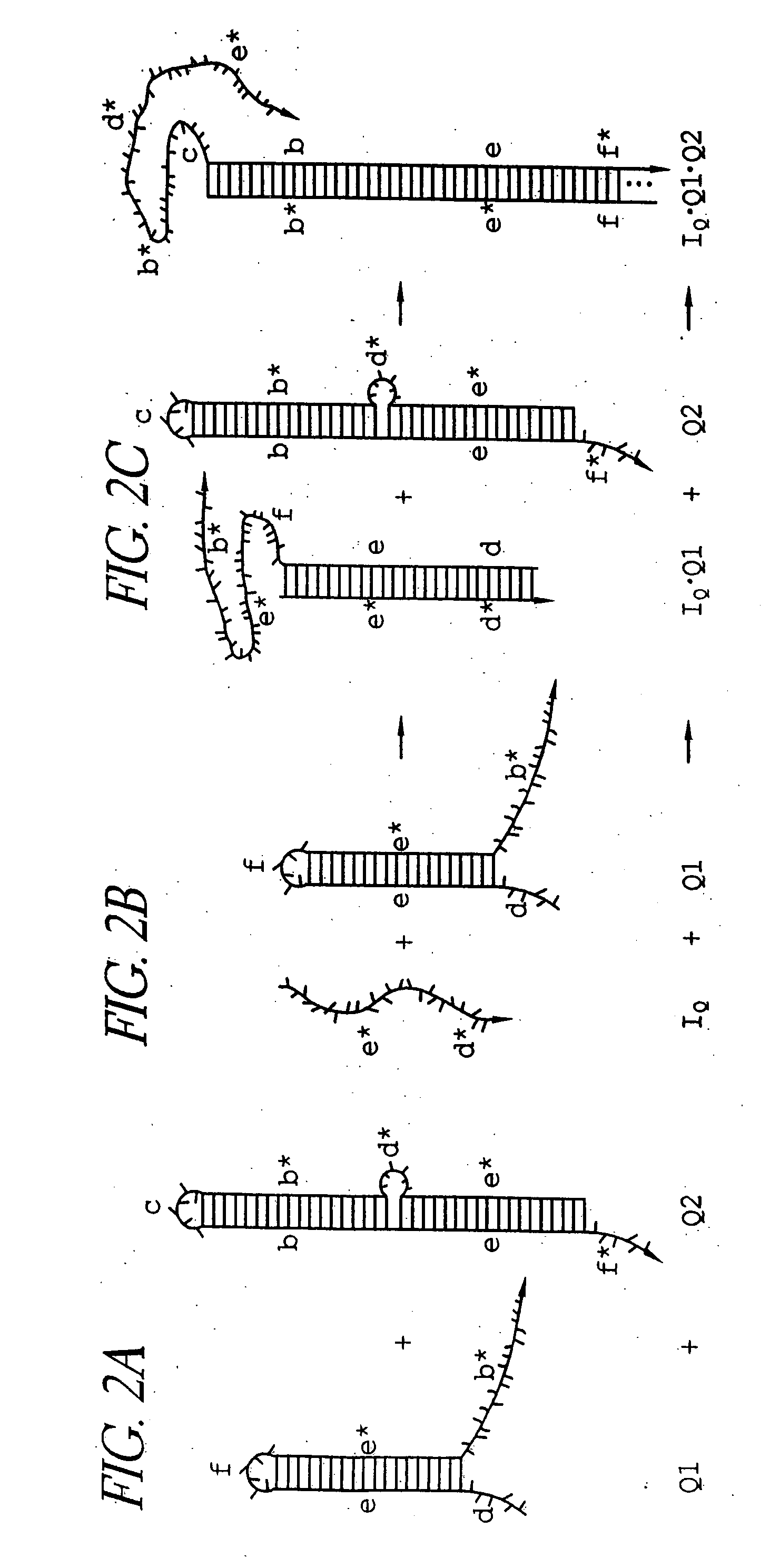
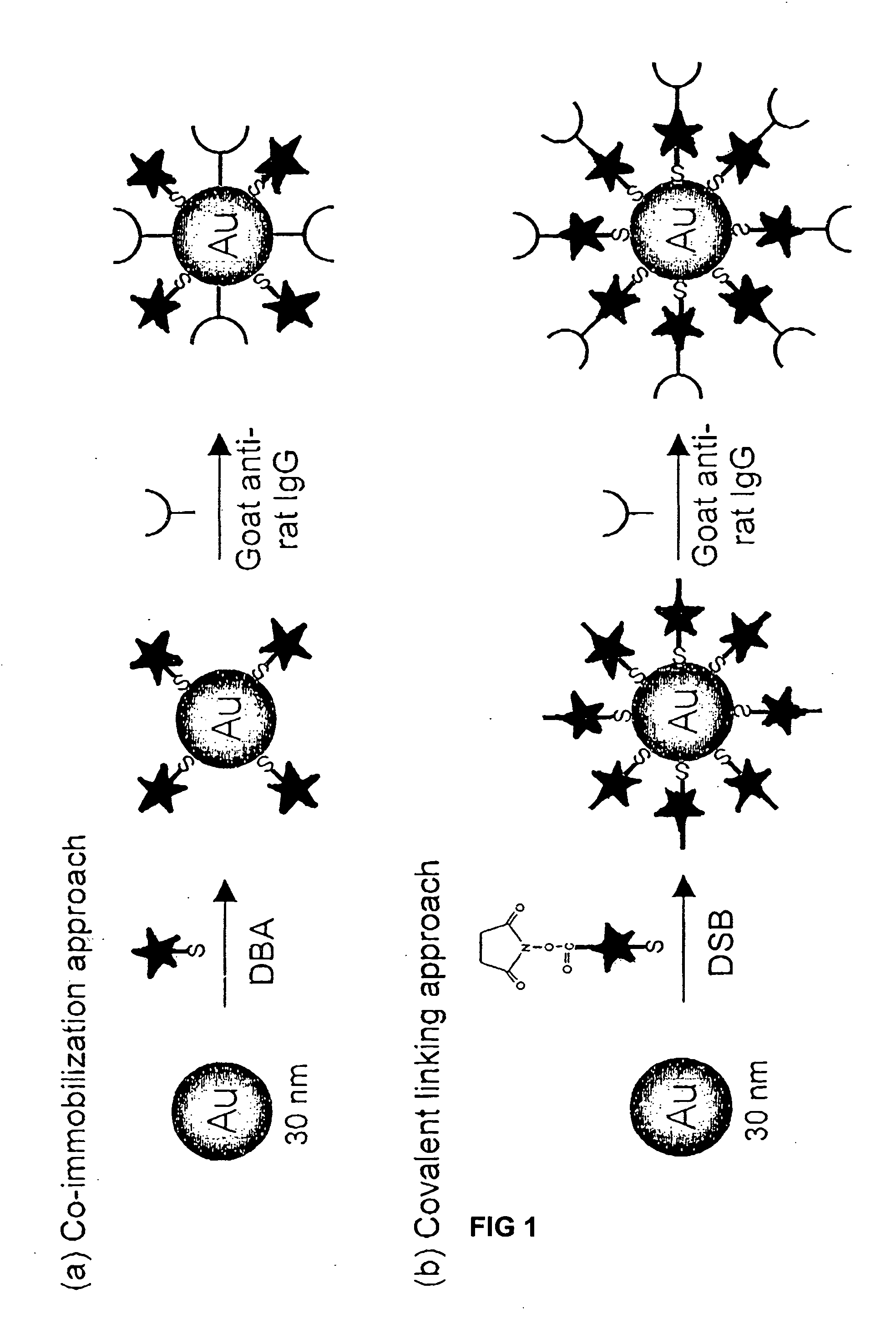
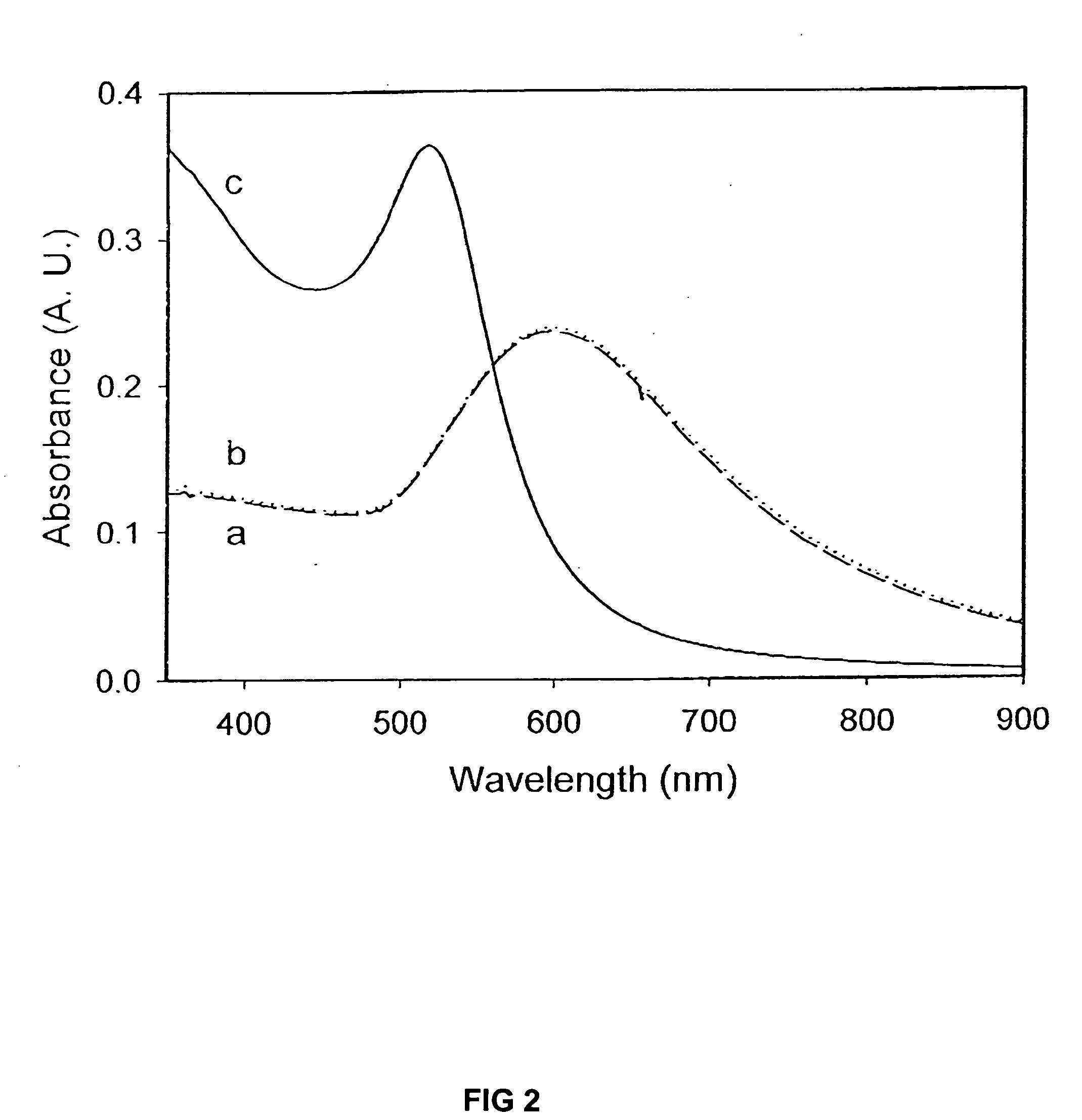
Patents
Literature
2006 results about "Fluorescent labelling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
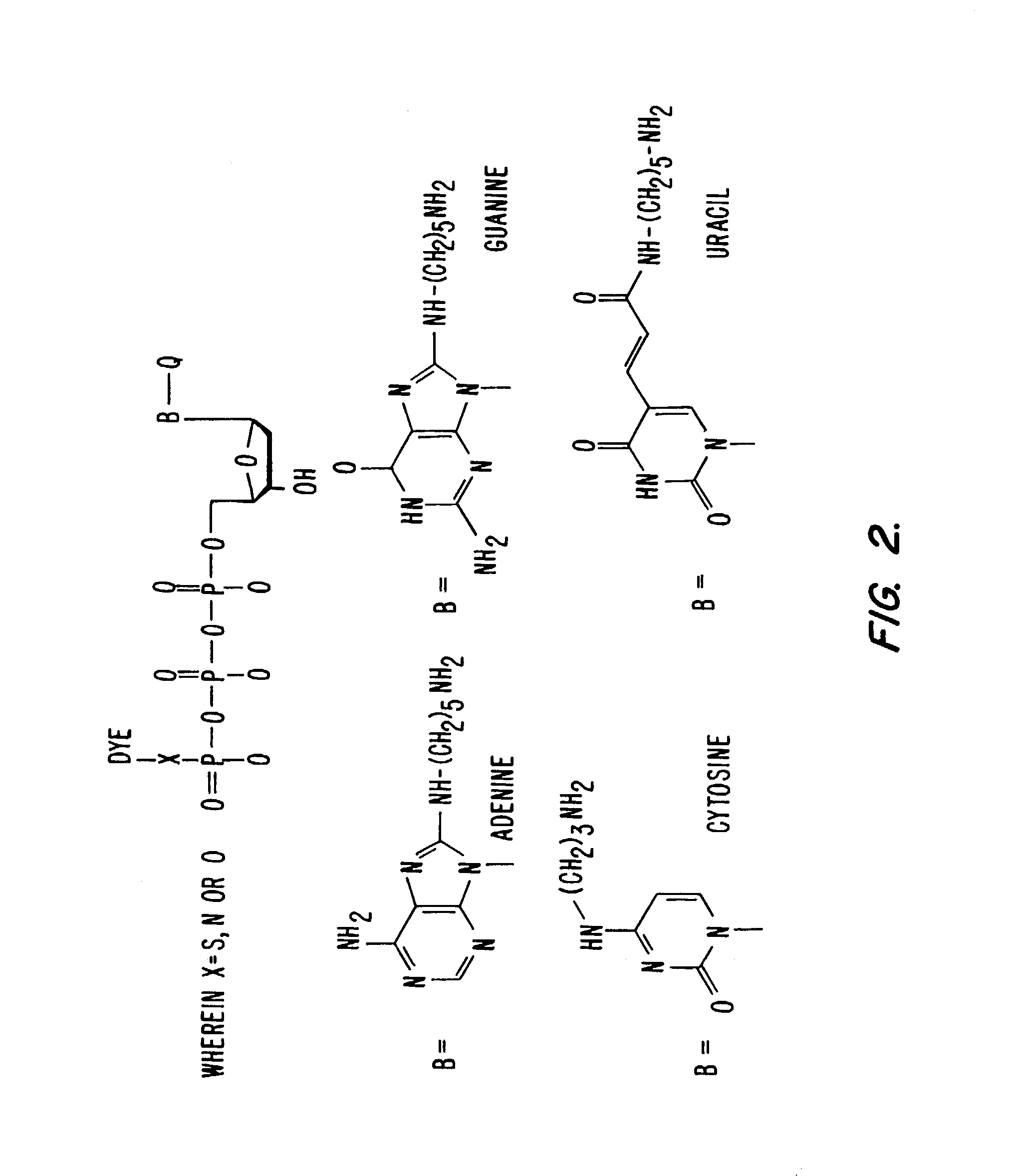
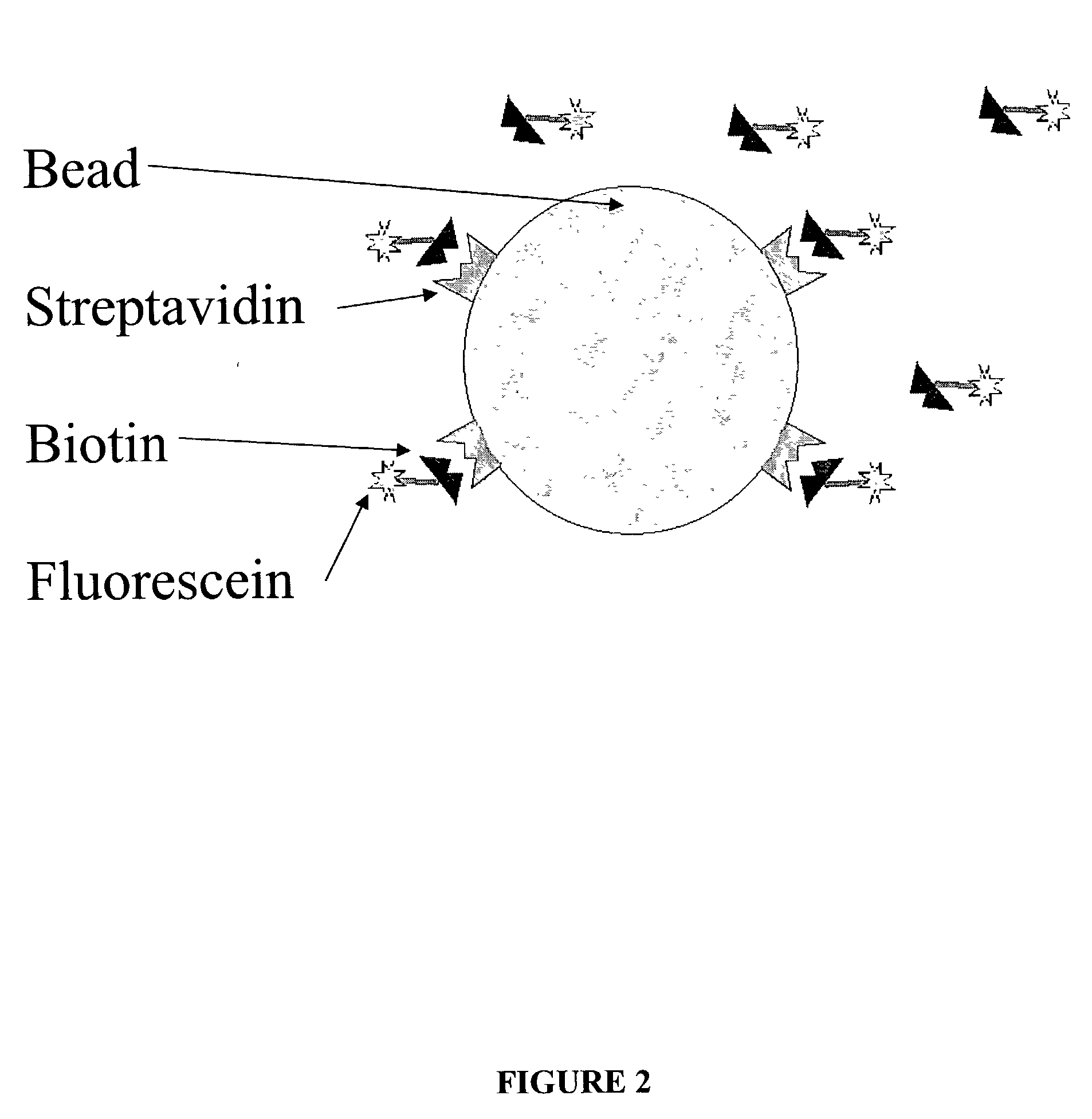
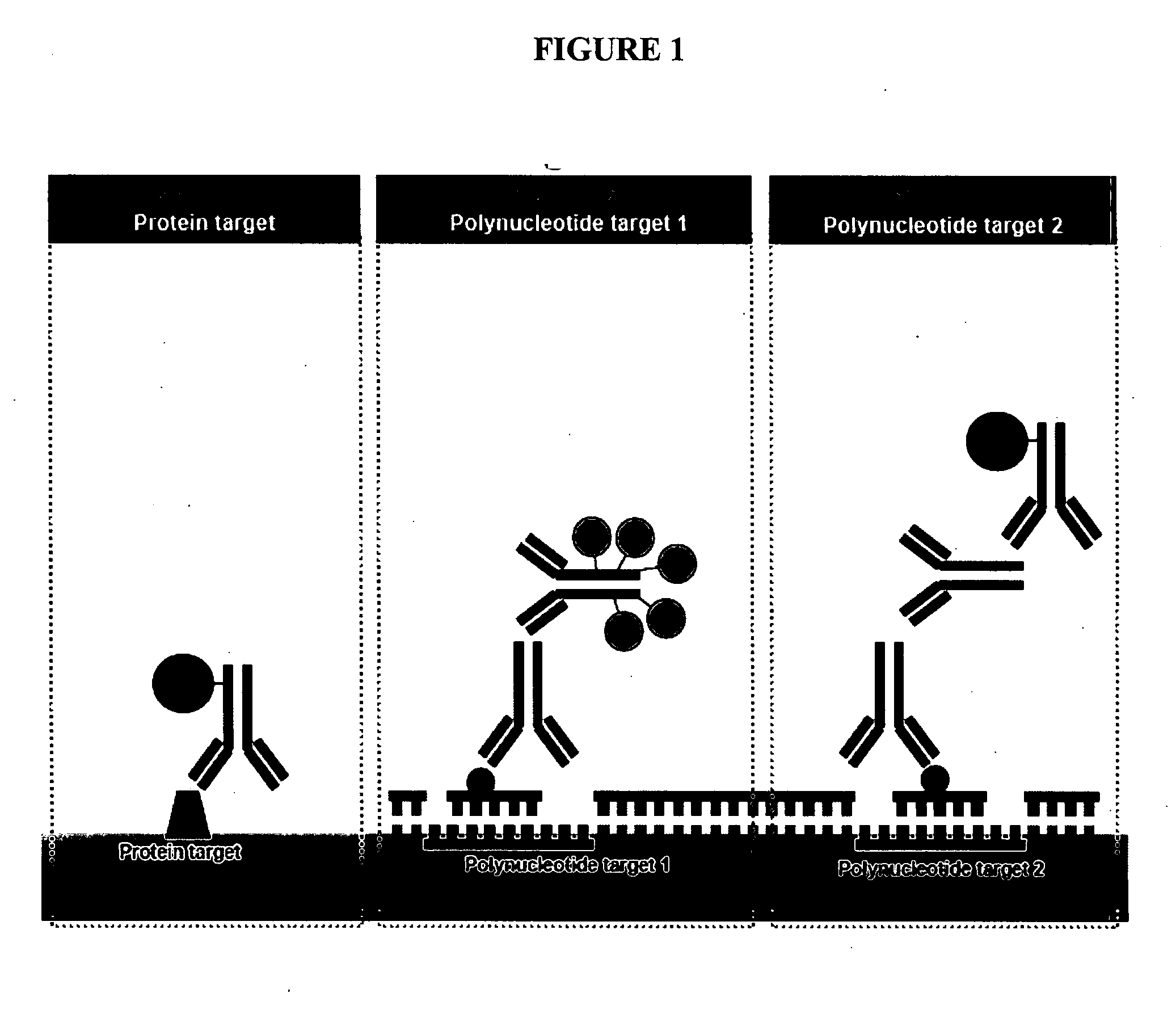
Fluorescent labelling is the process of covalently attaching a fluorophore to another molecule, such as a protein or nucleic acid. This is generally accomplished using a reactive derivative of the fluorophore that selectively binds to a functional group contained in the target molecule. The most commonly labelled molecules are antibodies, proteins, amino acids and peptides which are then used as specific probes for detection of a particular target.
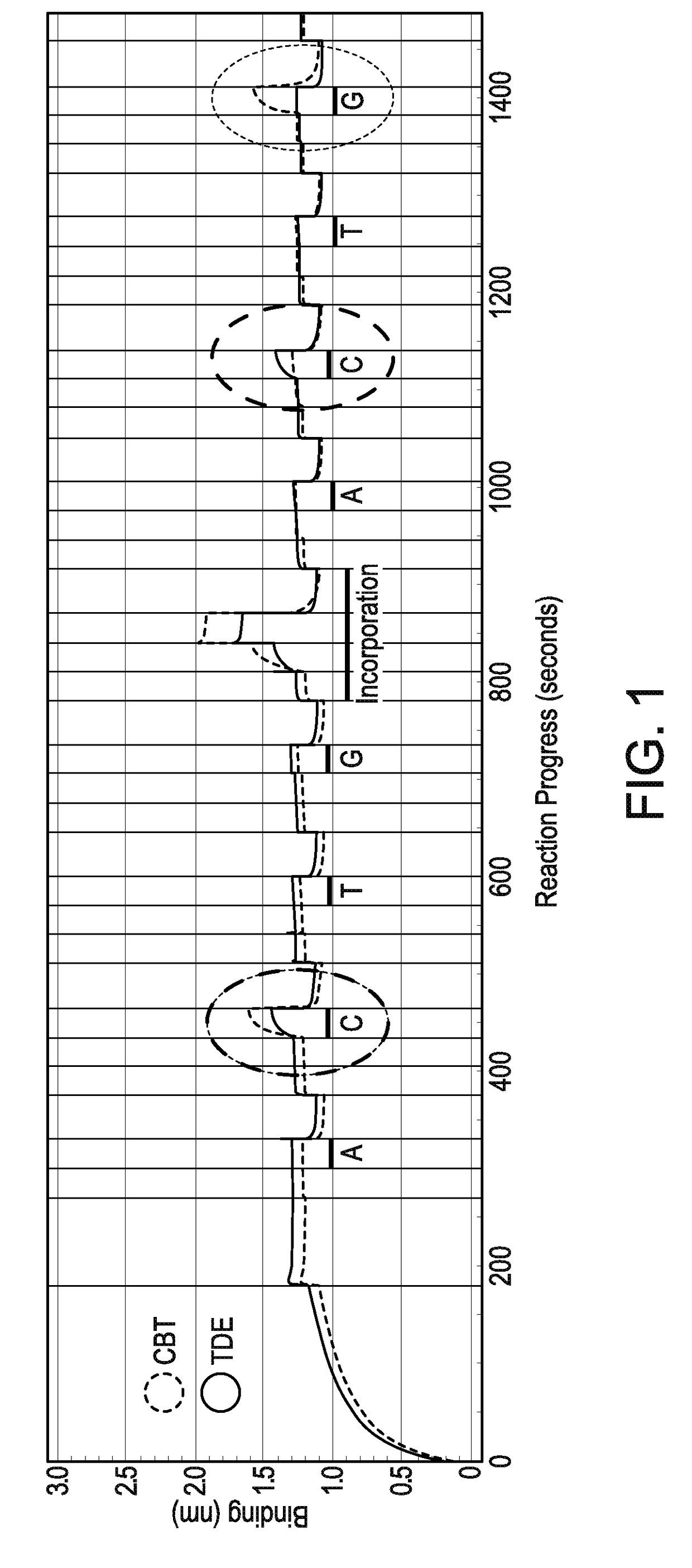
Sequencing by incorporation
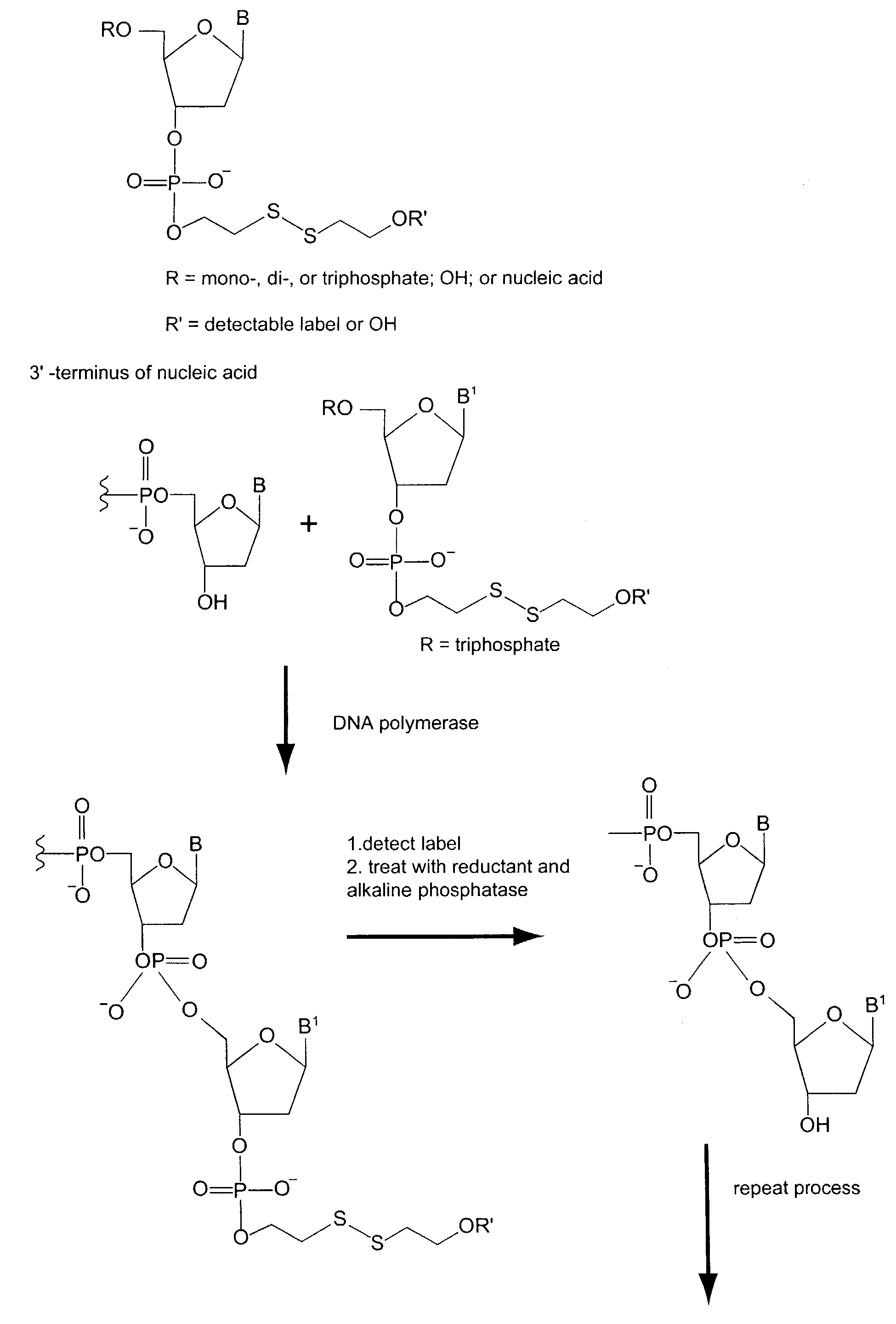
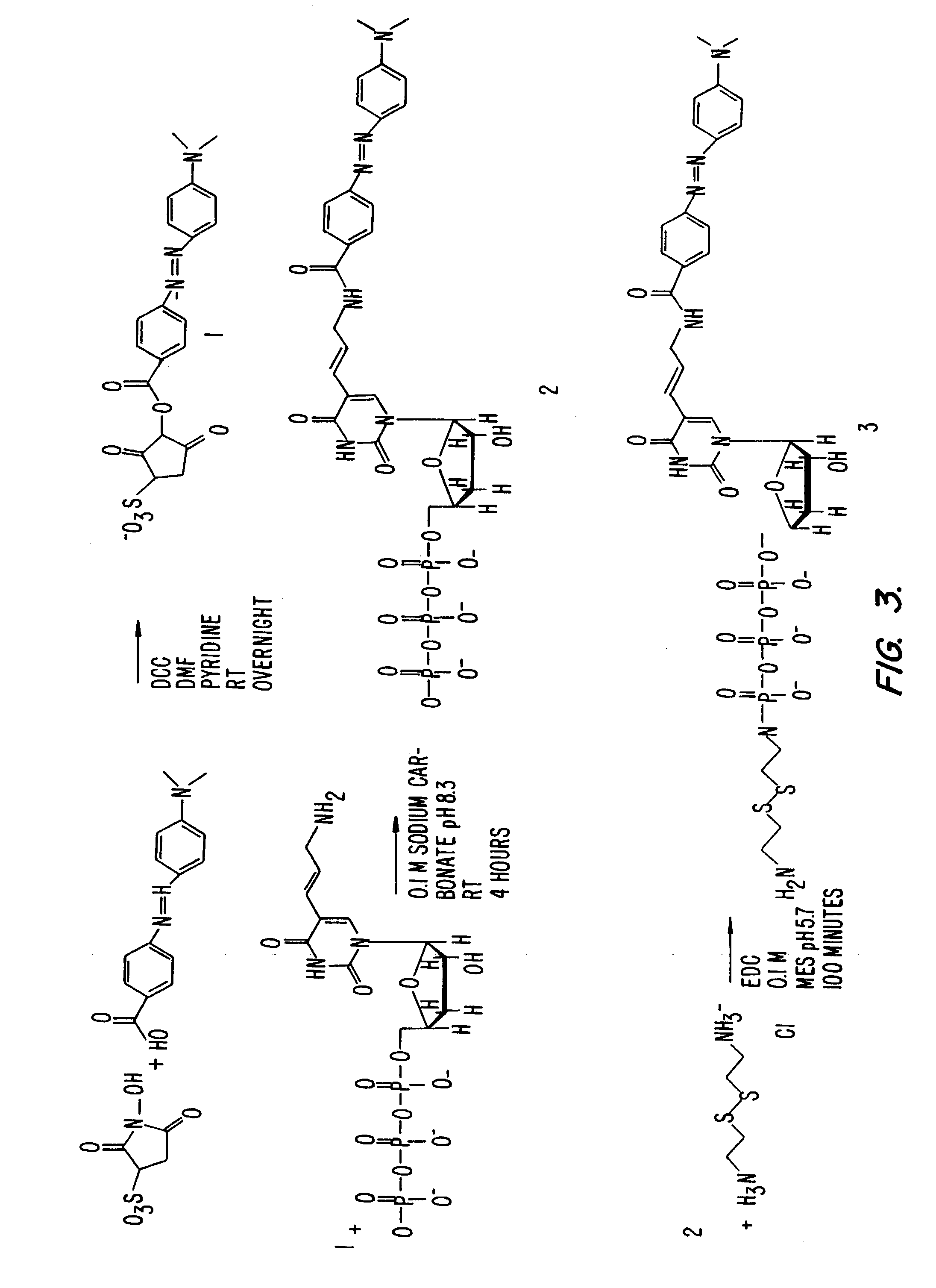
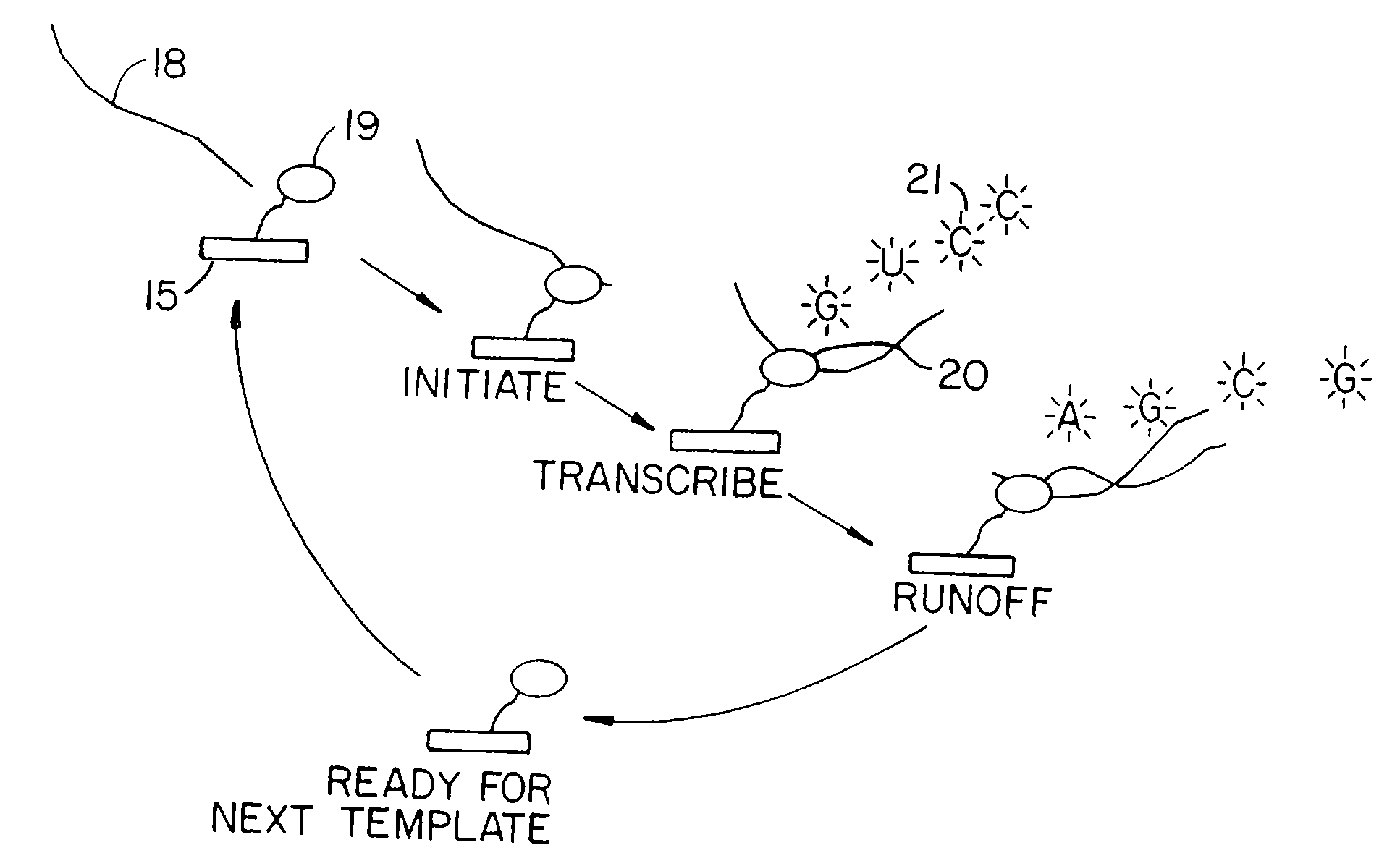
Nucleotides and nucleotide analogs are used in various sequencing by incorporation / sequencing by synthesis methods. Nucleotide analogs comprising 3′-blocking groups are used to provide reversible chain-termination for sequencing by synthesis. Typical blocking groups include phosphate groups and carbamate groups. Fluorescent nucleotides are used to perform sequencing by synthesis with detection by incorporation of the fluorescently labeled nucleotide, optionally followed by photobleaching and intercalating dyes are used to detect addition of a non-labeled nucleotide in sequencing by synthesis with detection by intercalation. Microfluidic devices, including particle arrays, are used in the sequencing methods.
Owner:CAPLIPER LIFE SCI INC
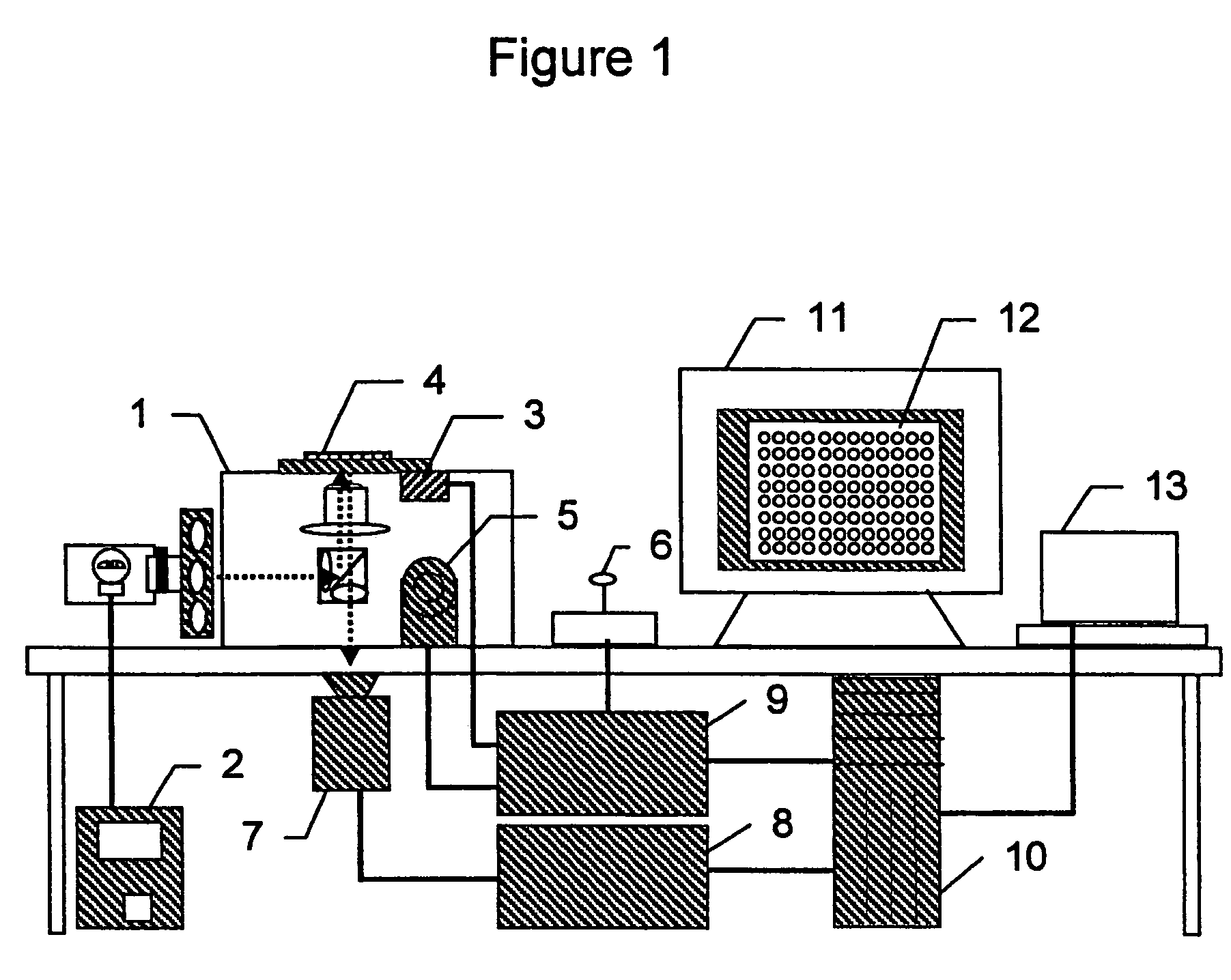
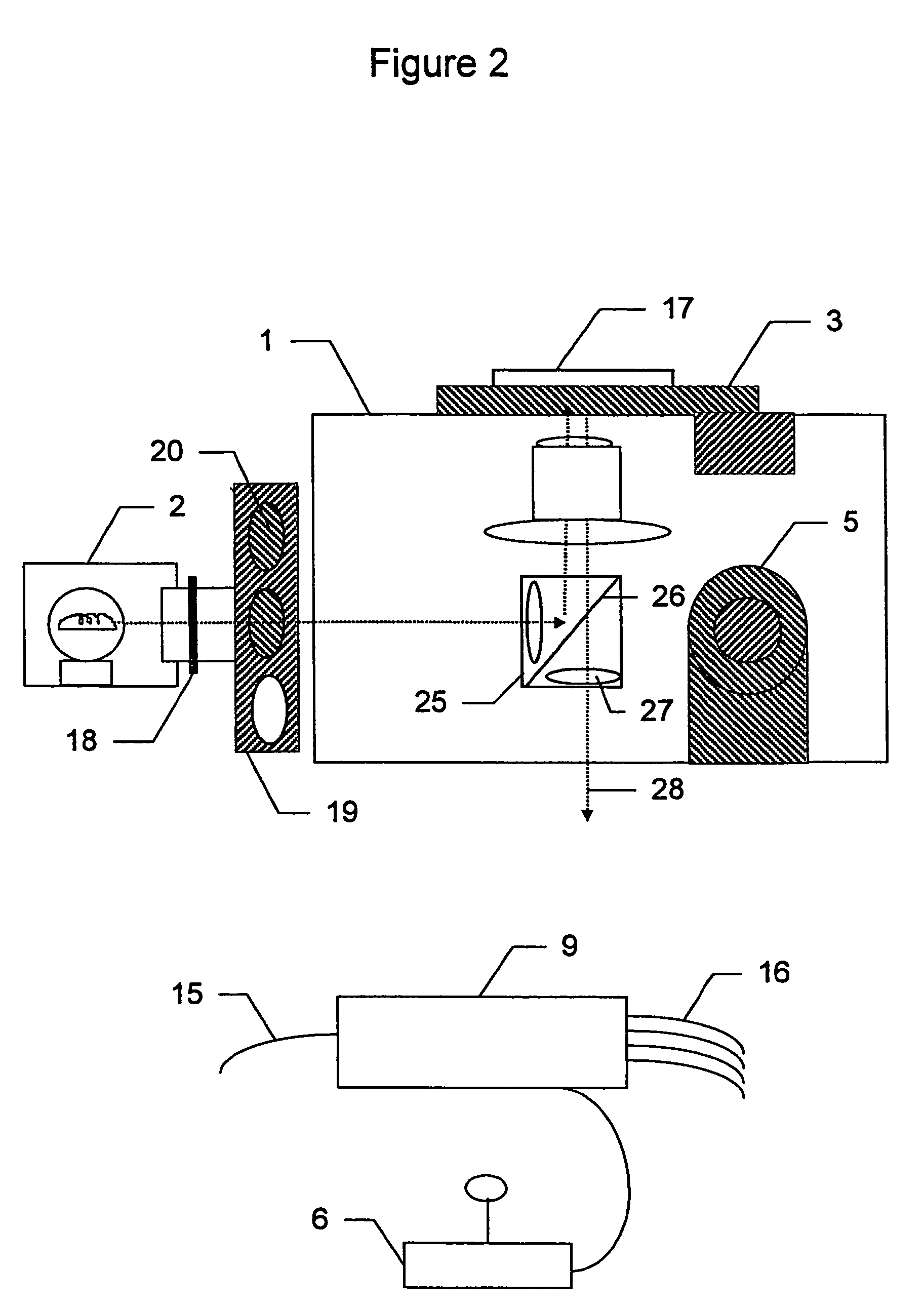
System and method for nucleic acid sequencing by polymerase synthesis
InactiveUS7229799B2Bioreactor/fermenter combinationsBiological substance pretreatmentsPolymerase LNucleic acid sequencing
This invention relates to improved methods for sequencing and genotyping nucleic acid in a single molecule configuration. The method involves single molecule detection of fluorescent labeled PPi moieties released from NTPs as a polymerase extension product is created.
Owner:PACIFIC BIOSCIENCES
Methods and algorithms for cell enumeration in low-cost cytometer
InactiveUS20060024756A1Simple designReduce operating costsBioreactor/fermenter combinationsBiological substance pretreatmentsWhite blood cellCcd camera
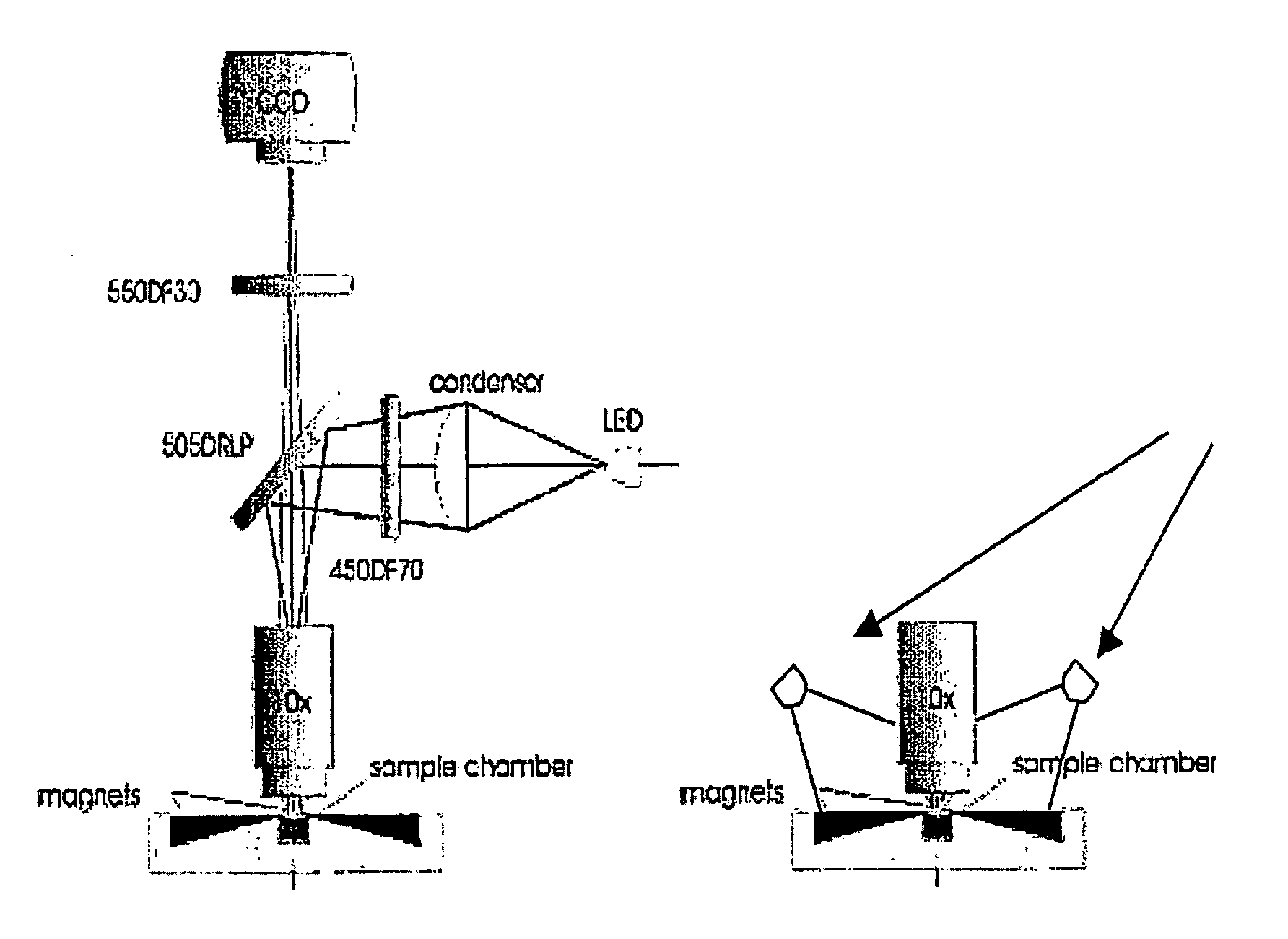
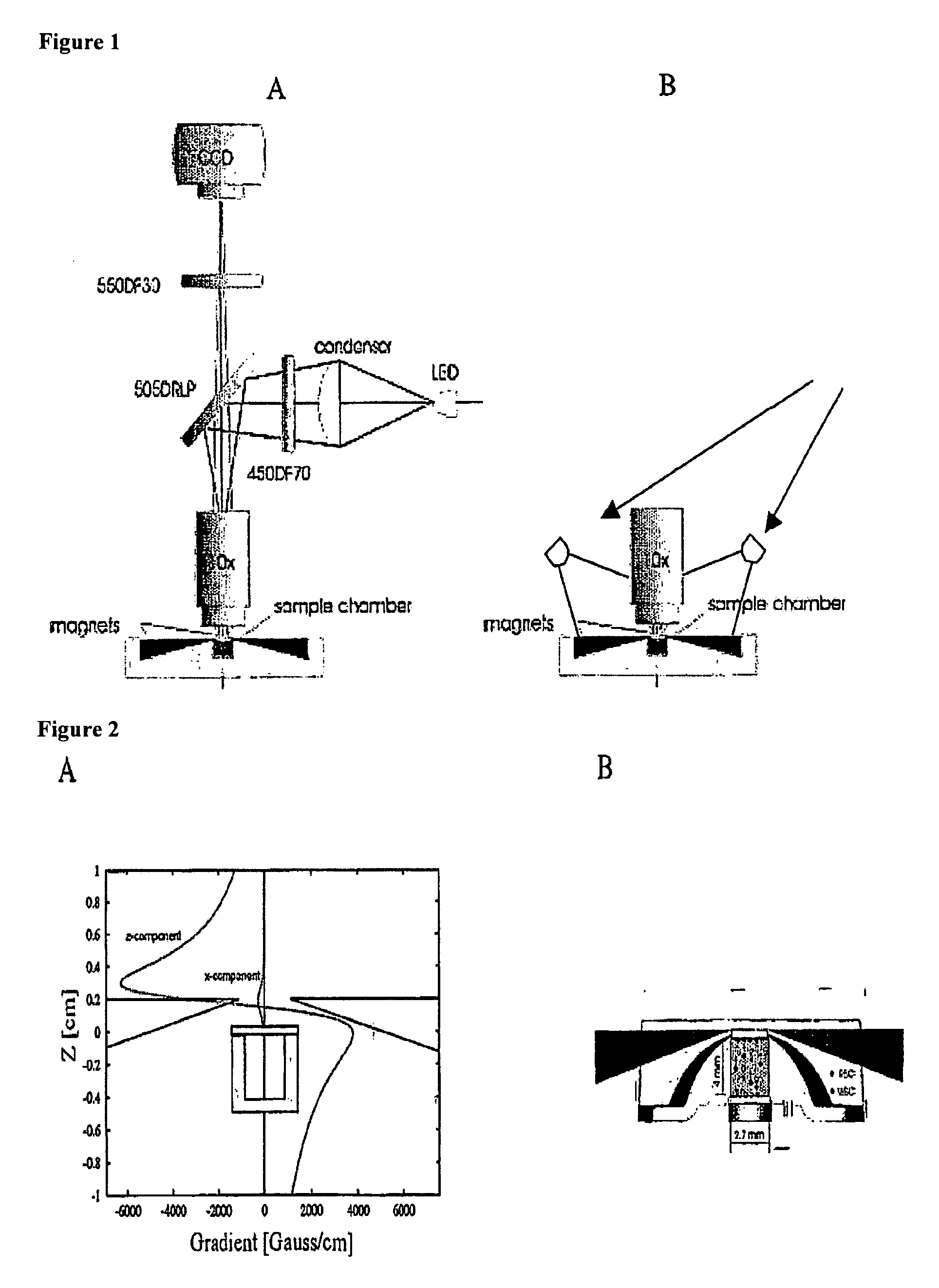
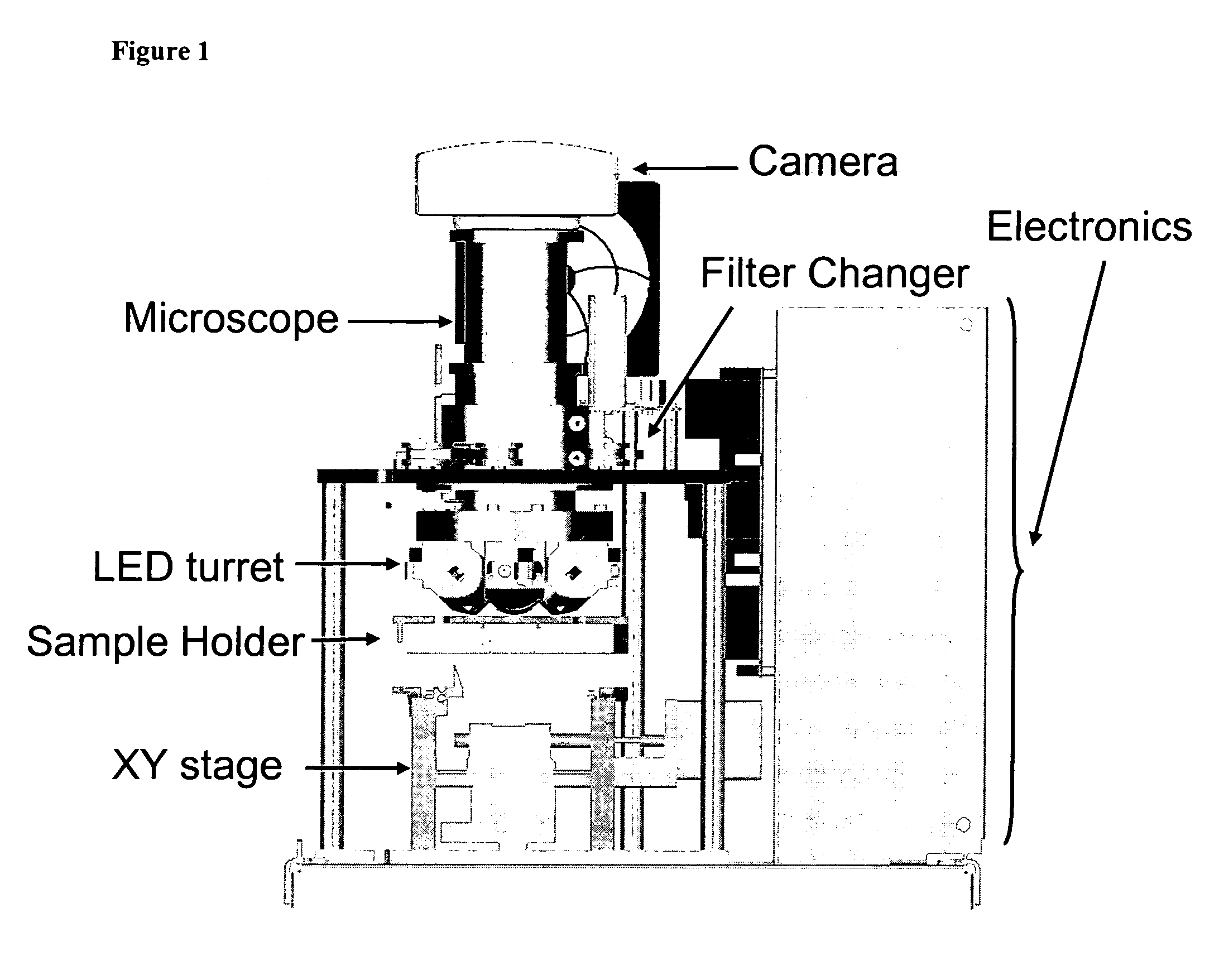
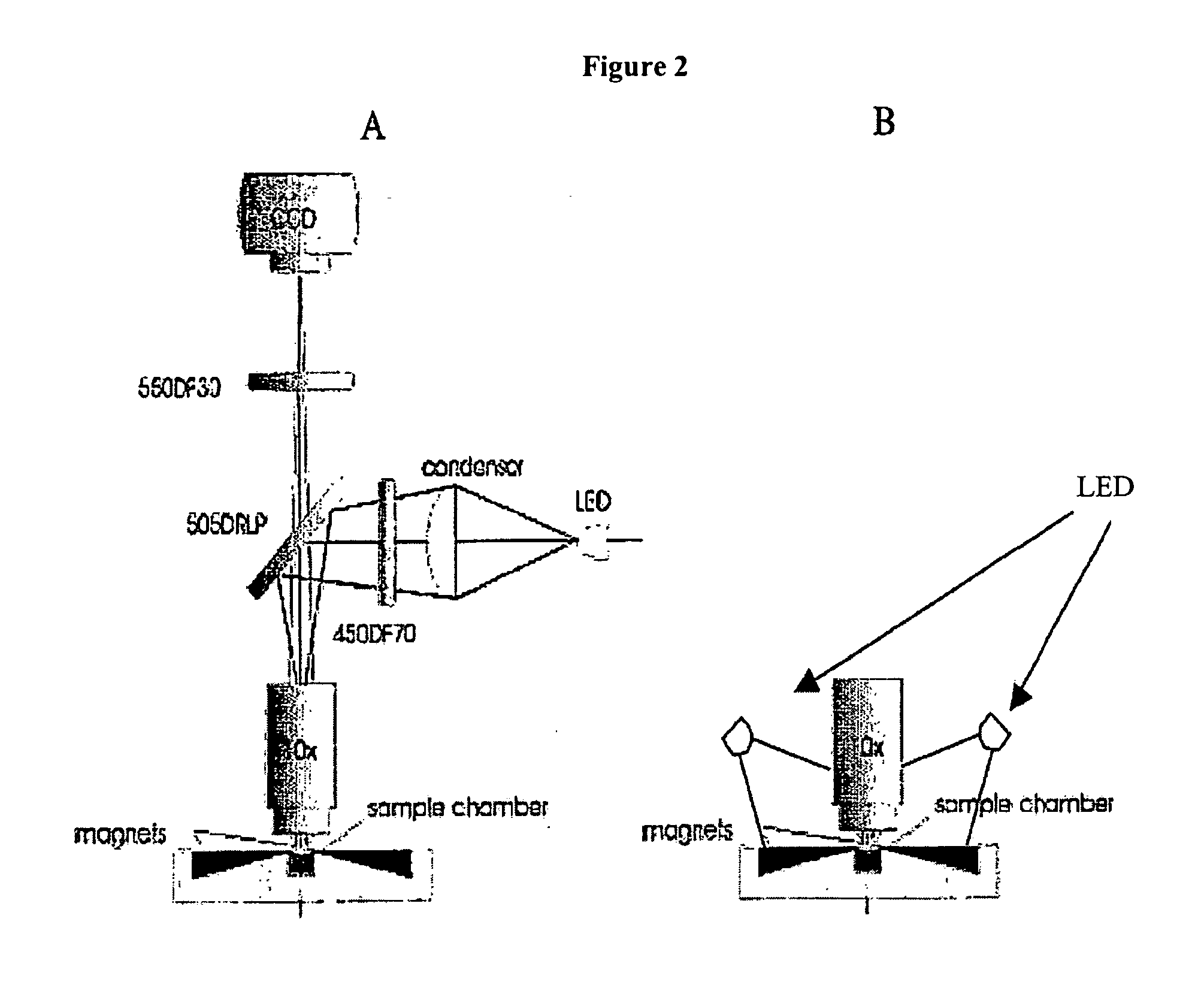
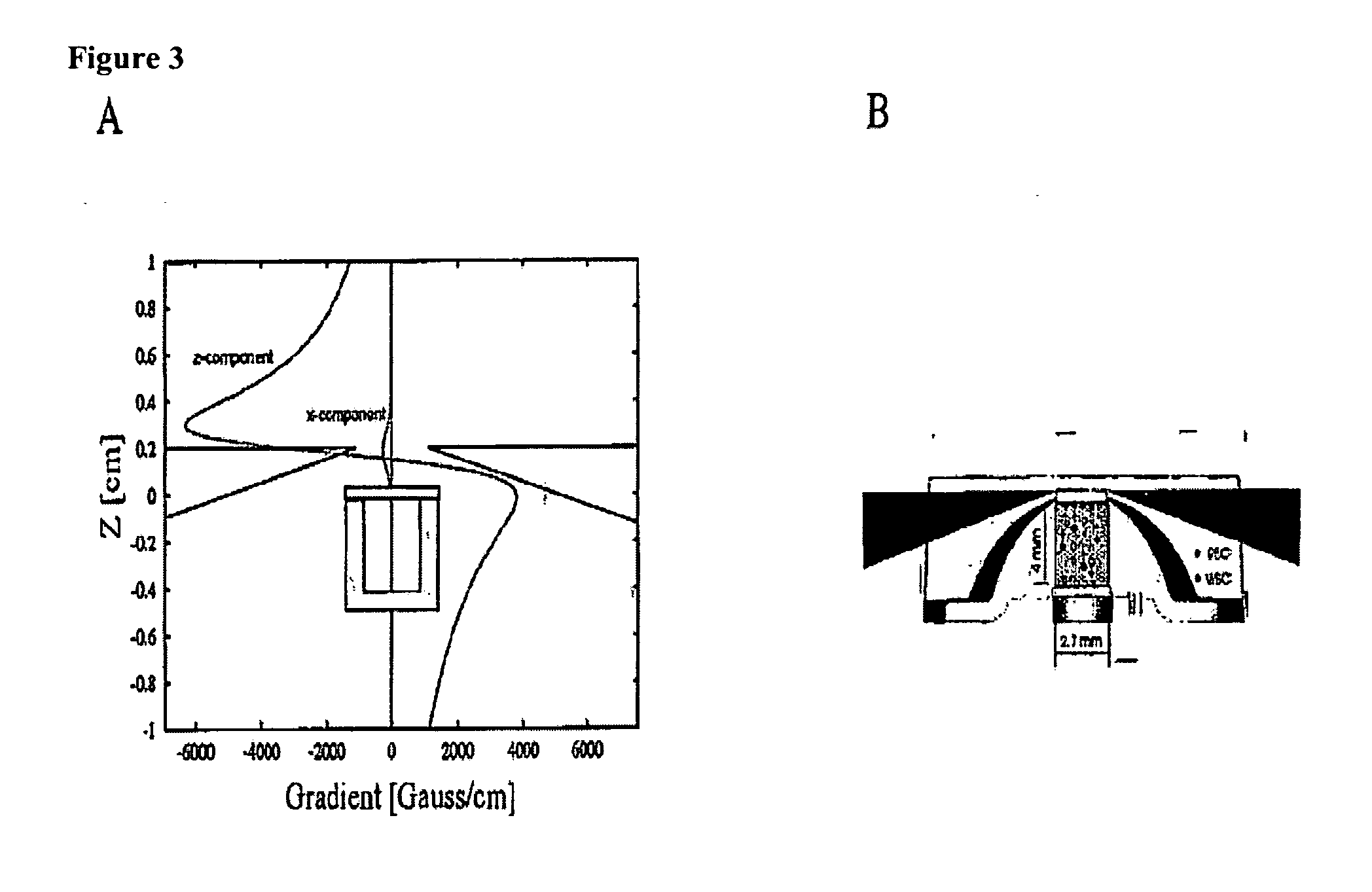
The enumeration of cells in fluids by flow cytometry is widely used across many disciplines such as assessment of leukocyte subsets in different bodily fluids or of bacterial contamination in environmental samples, food products and bodily fluids. For many applications the cost, size and complexity of the instruments prevents wider use, for example, CD4 analysis in HIV monitoring in resource-poor countries. The novel device, methods and algorithms disclosed herein largely overcome these limitations. Briefly, all cells in a biological sample are fluorescently labeled, but only the target cells are also magnetically labeled. The labeled sample, in a chamber or cuvet, is placed between two wedge-shaped magnets to selectively move the magnetically labeled cells to the observation surface of the cuvet. An LED illuminates the cells and a CCD camera captures the images of the fluorescent light emitted by the target cells. Image analysis performed with a novel algorithm provides a count of the cells on the surface that can be related to the target cell concentration of the original sample. The compact cytometer system provides a rugged, affordable and easy-to-use technique, which can be used in remote locations.
Owner:UNIVERSITY OF TWENTE
Dual mode real-time screening and rapid full-area, selective-spectral, remote imaging and analysis device and process
InactiveUS20030158470A1Quick scanAvoid delayDiagnostics using spectroscopyEndoscopesSpectral bandsImaging processing
The invention provides a device and process for real-time screening of areas that can be identified as suspicious either through image segmentation utilizing image processing techniques or through treatment with an exogenous fluorescent marker that selectively localizes in abnormal areas. If screening detects a suspicious area, then the invention allows acquiring of autofluorescence images at multiple selected narrow differentiating spectral bands so that a "virtual biopsy" can be obtained to differentiate abnormal areas from normal areas based on differentiating portions of autofluorescence spectra. Full spatial information is collected, but autofluorescence data is collected only at the selected narrow spectral bands, avoiding the collection of full spectral data, so that the speed of analysis is increased.
Owner:STI MEDICAL SYST
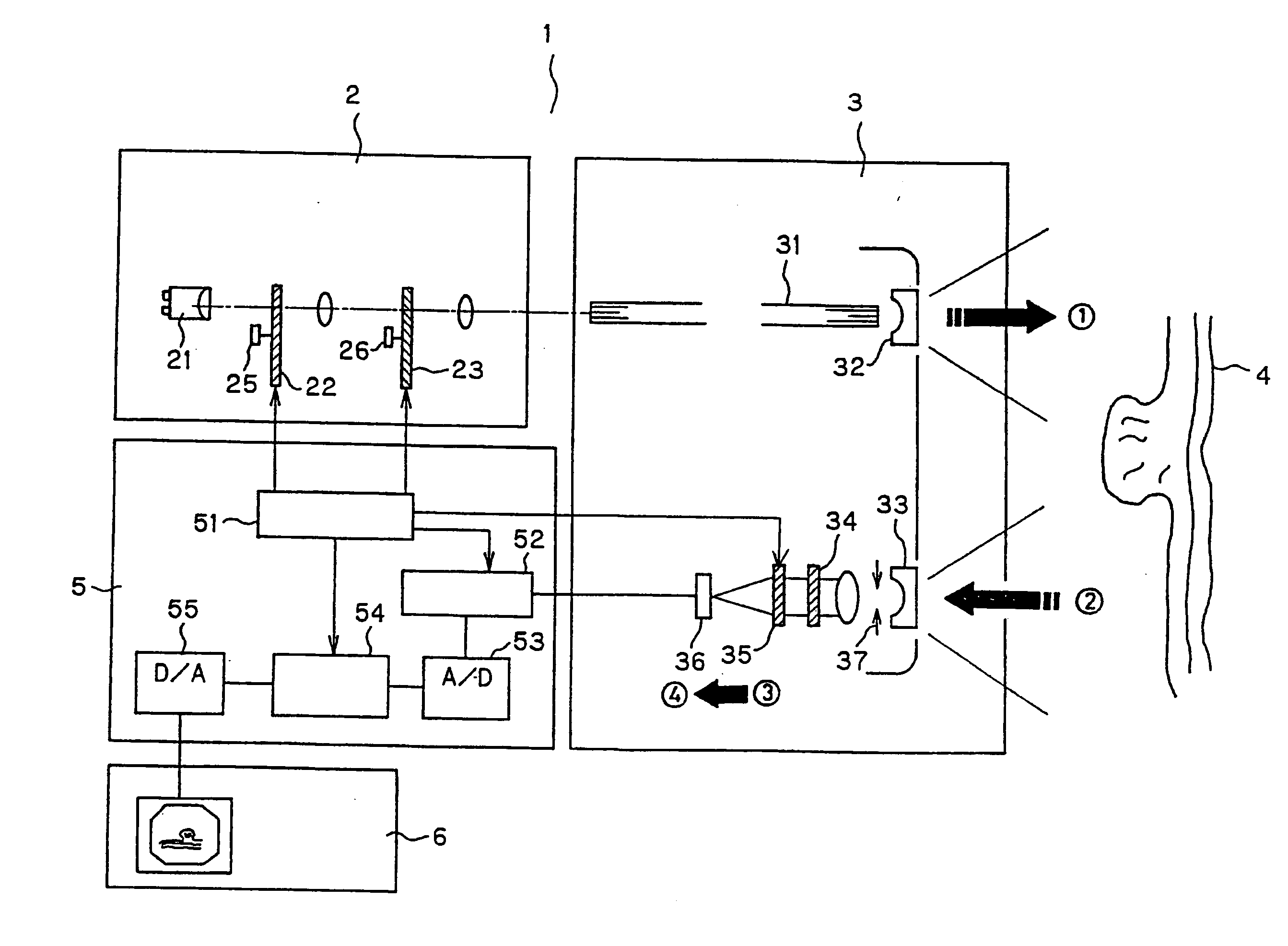
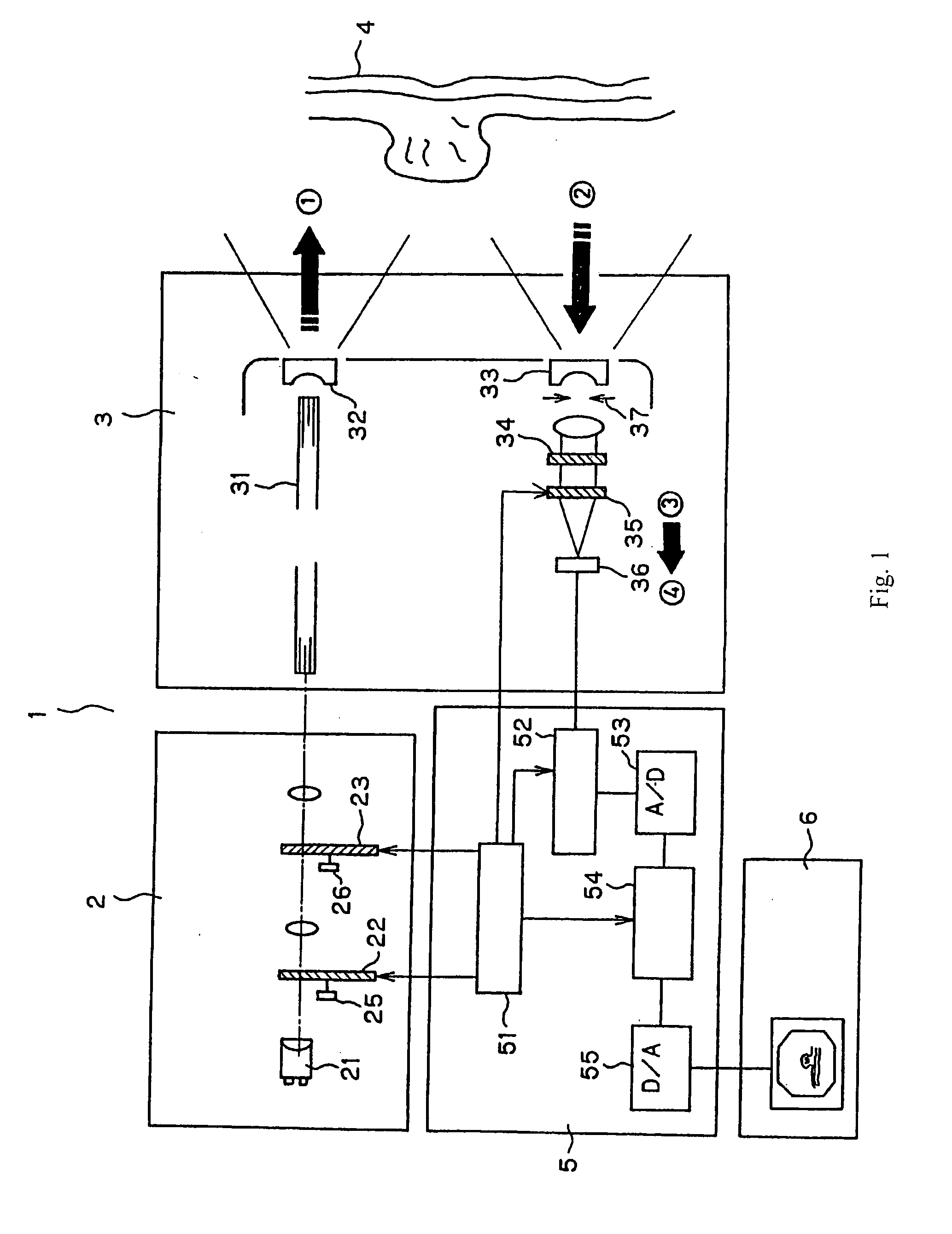
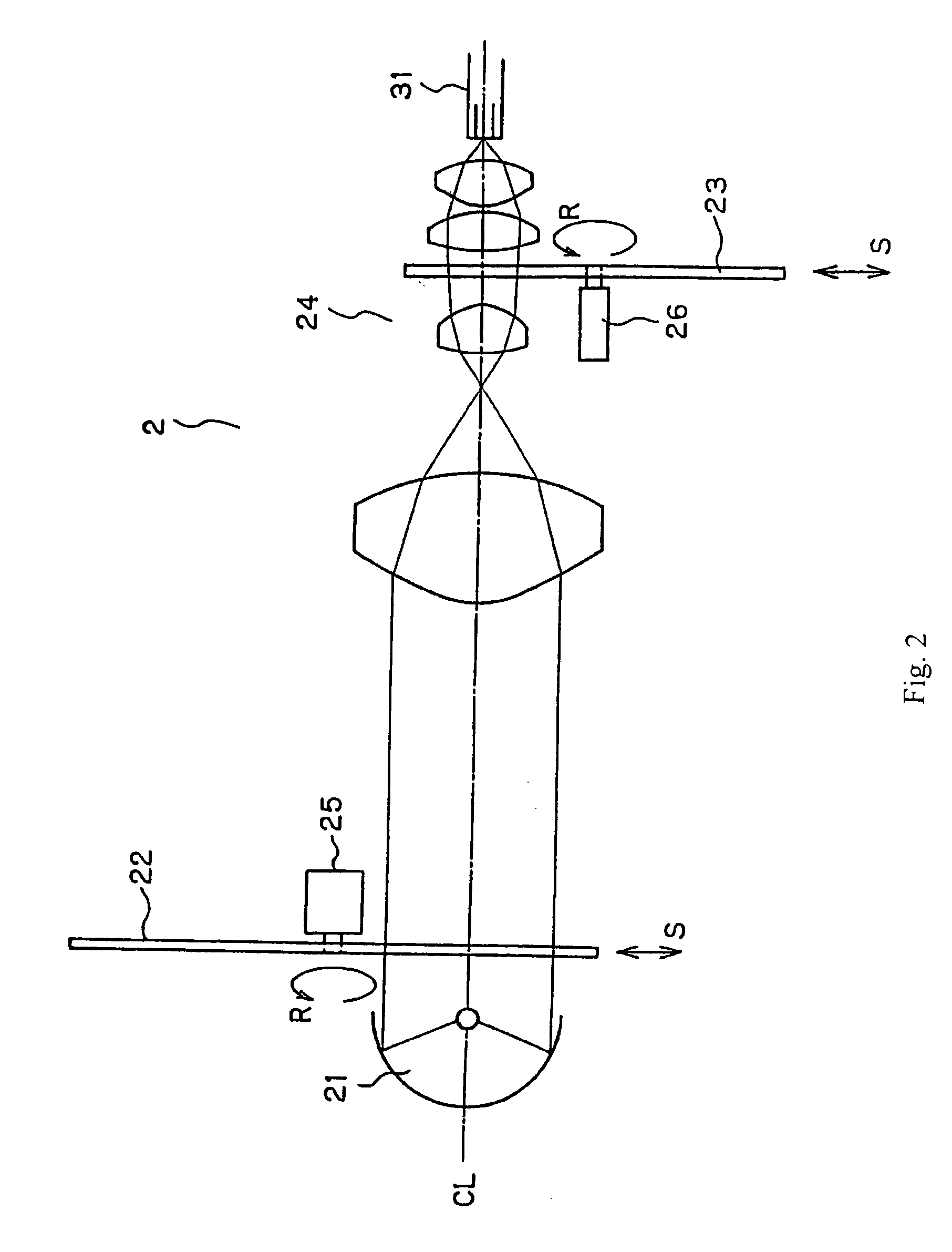
Endoscope system for fluorescent observation
An endoscope system is disclosed for detecting fluorescent light emitted in the near-infrared region by a plurality of fluorescent labeling materials introduced into a living tissue. An illumination system generates illumination light in the wavelength range 600 nm-2000 nm which serves as excitation light for the plurality of fluorescent labeling materials, and a detection system that can separately detect different ones of the plurality of fluorescent light emissions that are emitted at different wavelengths from among the plurality of fluorescent labeling materials is provided. The endoscope system may include a conventional-type endoscope having an insertion section, or a capsule endoscope that wirelessly transmits image data. By superimposing the image data obtained using reflected light in the visible region and fluorescent light emitted by the fluorescent labeling materials, improved diagnostic capabilities are provided.
Owner:OLYMPUS CORP
System and methods for nucleic acid sequencing of single molecules by polymerase synthesis
InactiveUS20090082212A1Bioreactor/fermenter combinationsBiological substance pretreatmentsPolymerase LNucleic acid sequencing
Owner:PACIFIC BIOSCIENCES
Modified nucleosides and nucleotides and uses thereof
ActiveUS7592435B2Increase brightnessHigh fluorescence intensitySugar derivativesMicrobiological testing/measurementNucleotidePurine
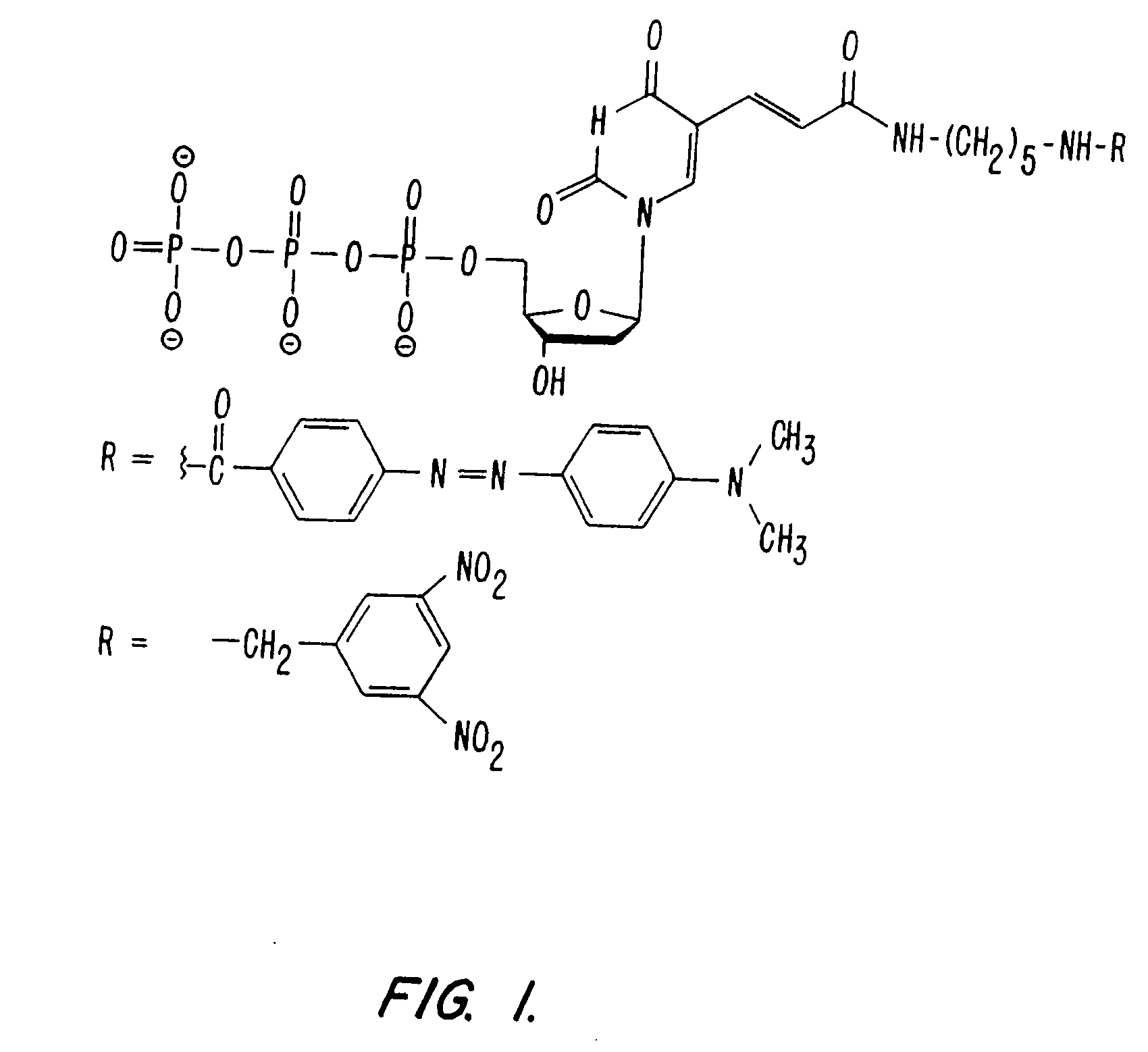
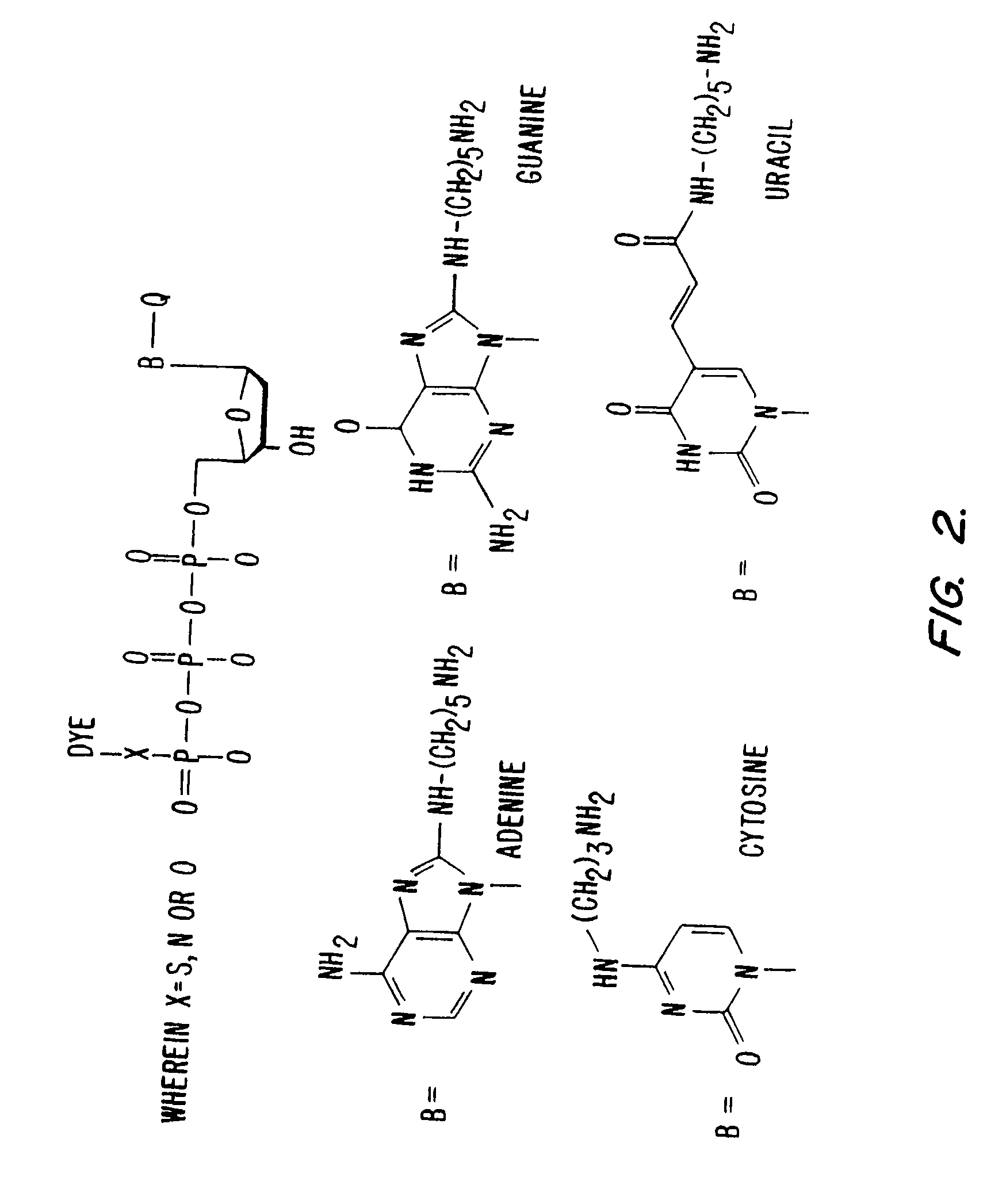
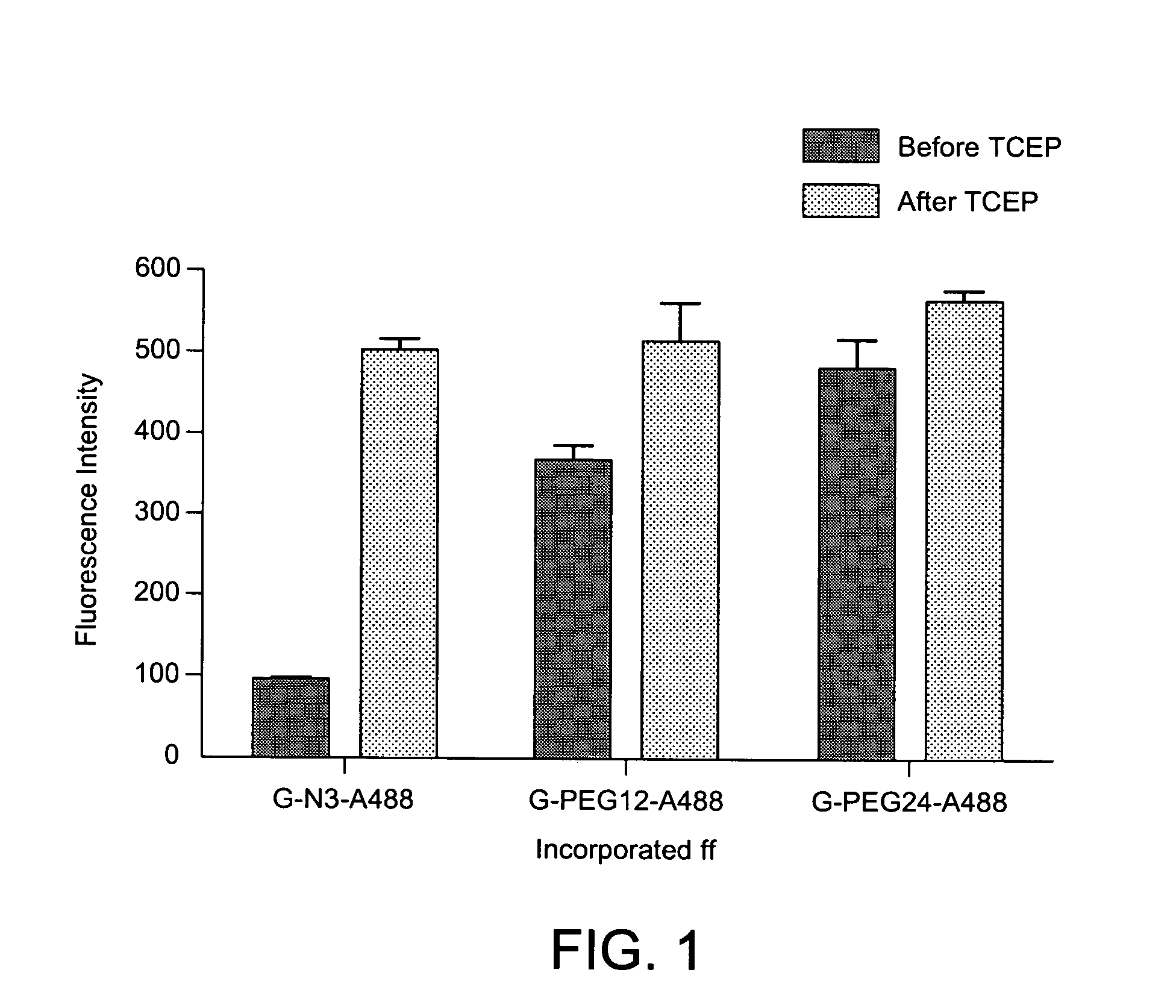
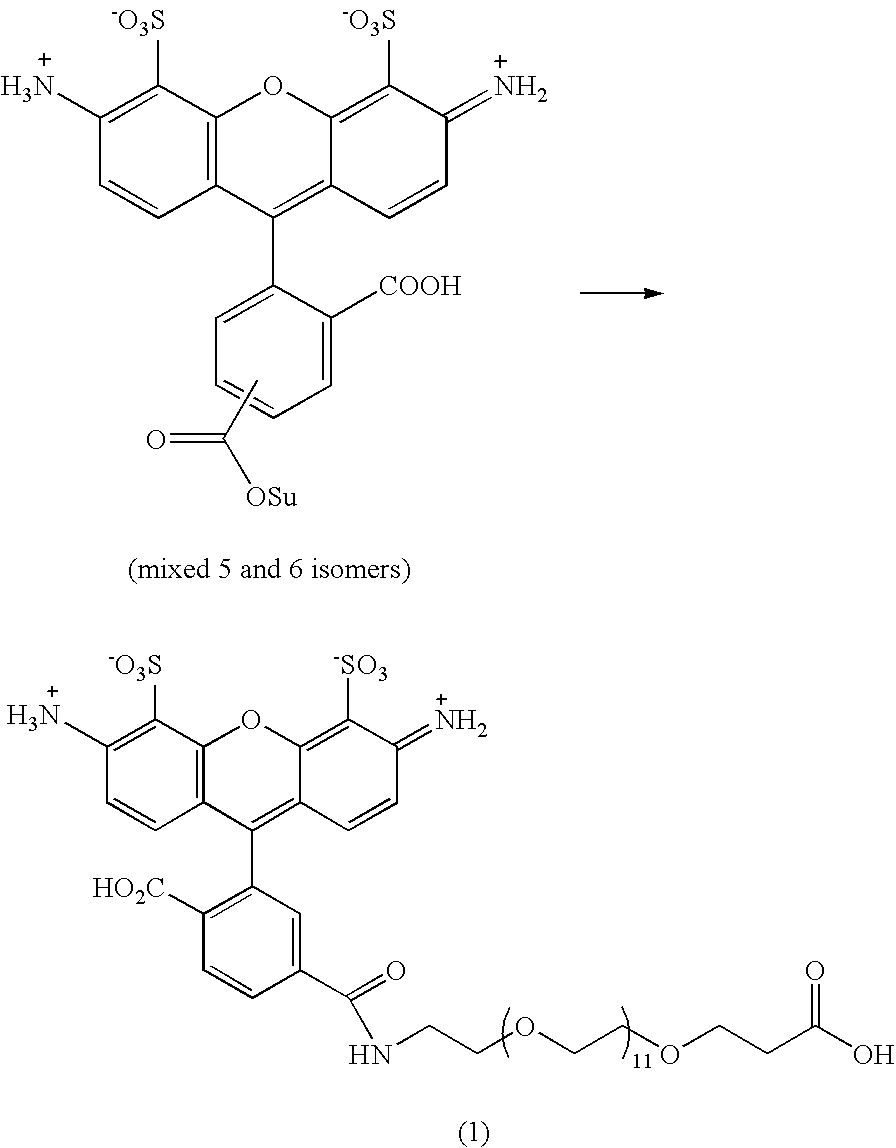
The invention is directed to modified guanine-containing nucleosides and nucleotides and uses thereof. More specifically, the invention relates to modified fluorescently labelled guanine-containing nucleosides and nucleotides which exhibit enhanced fluorophore intensity by virtue of reduced quenching effects.
Owner:ILLUMINA CAMBRIDGE LTD
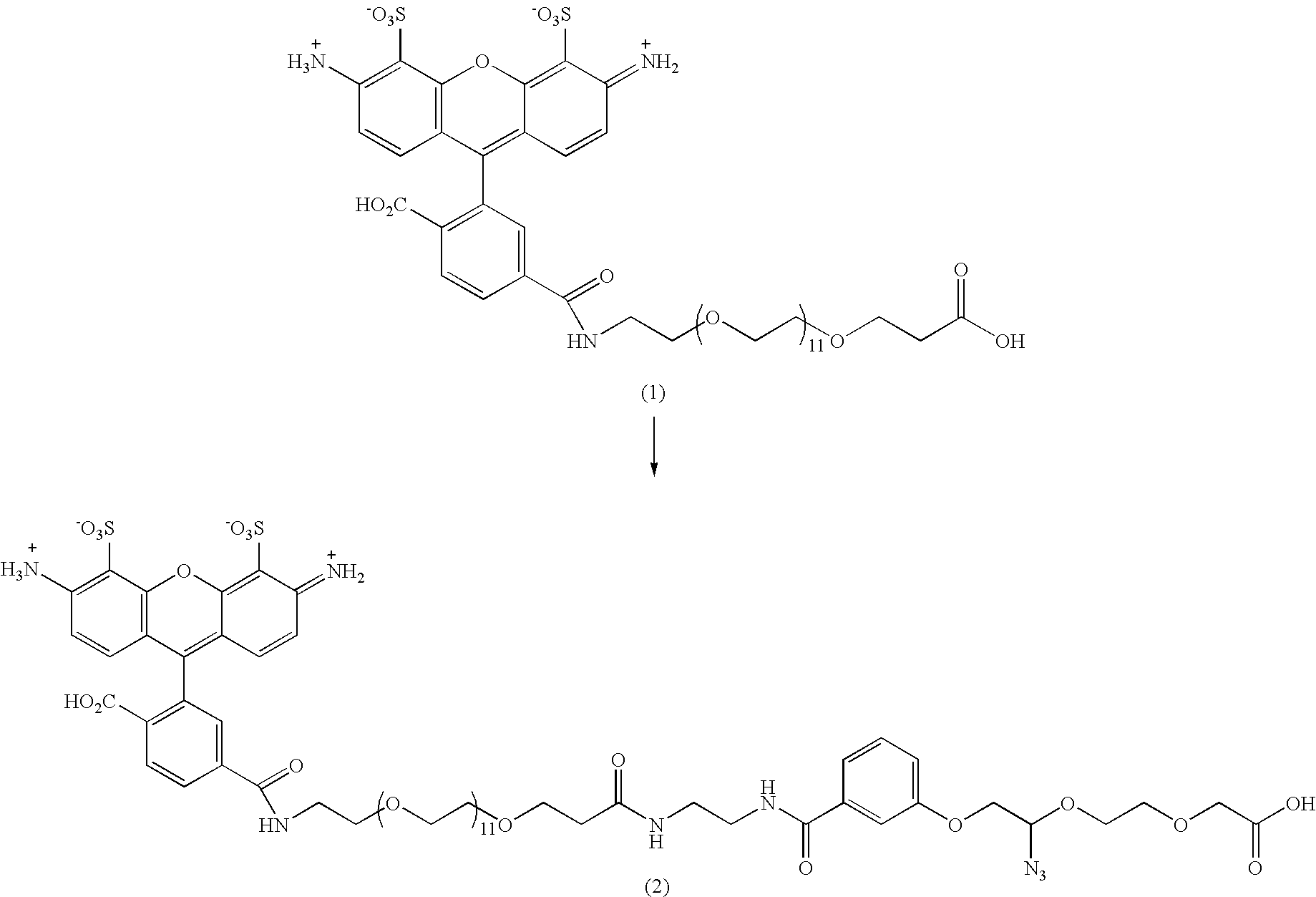
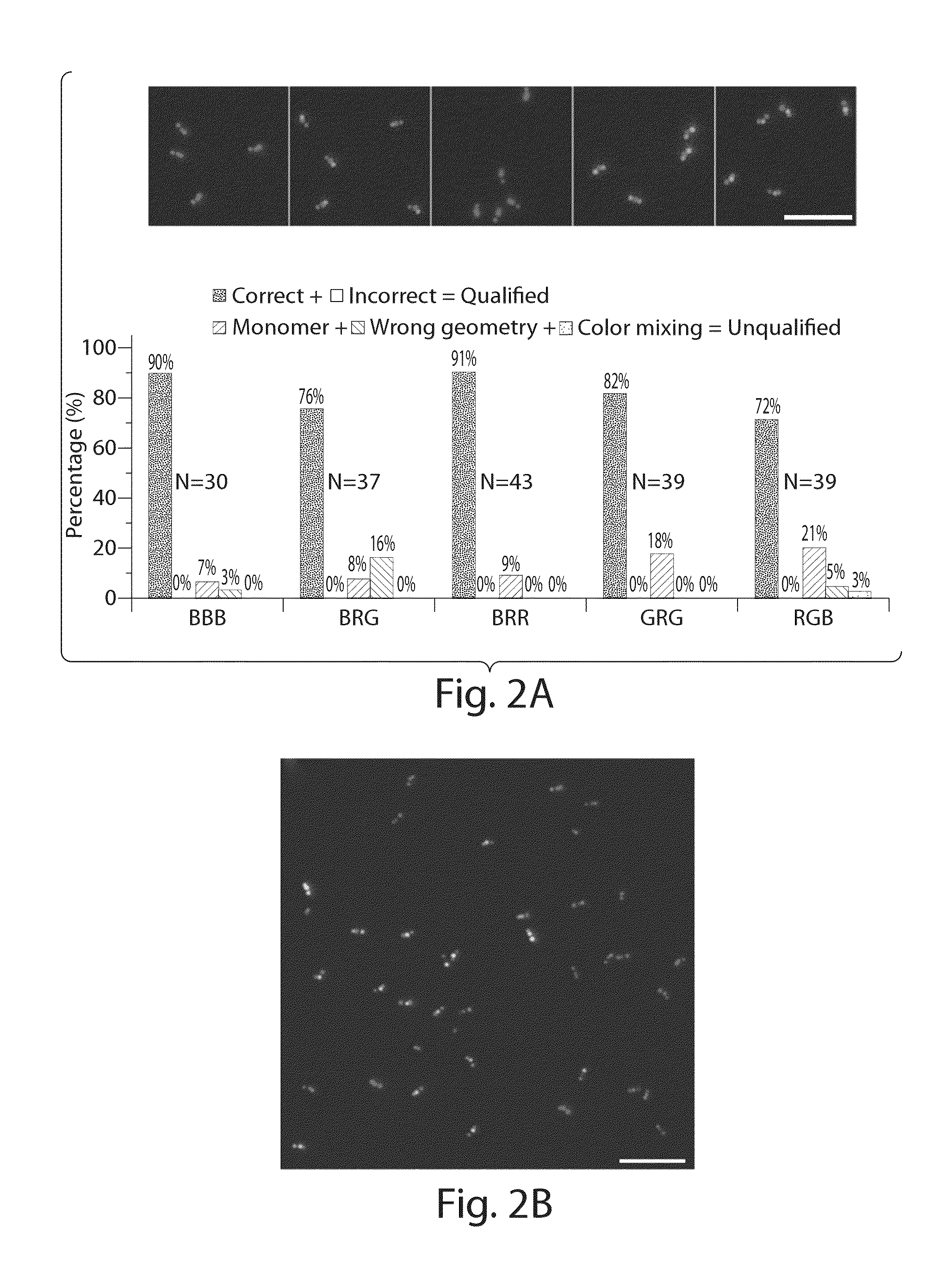
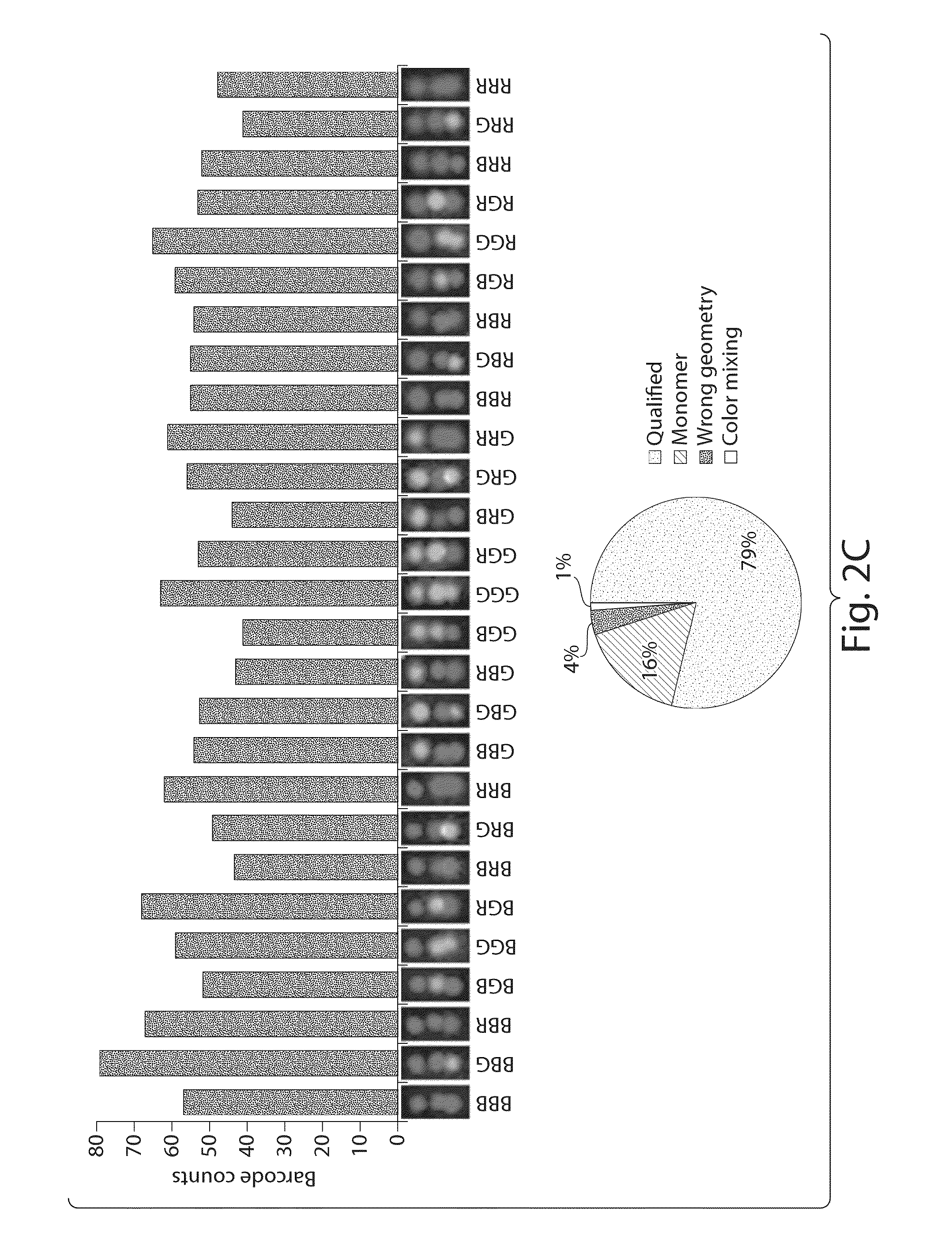
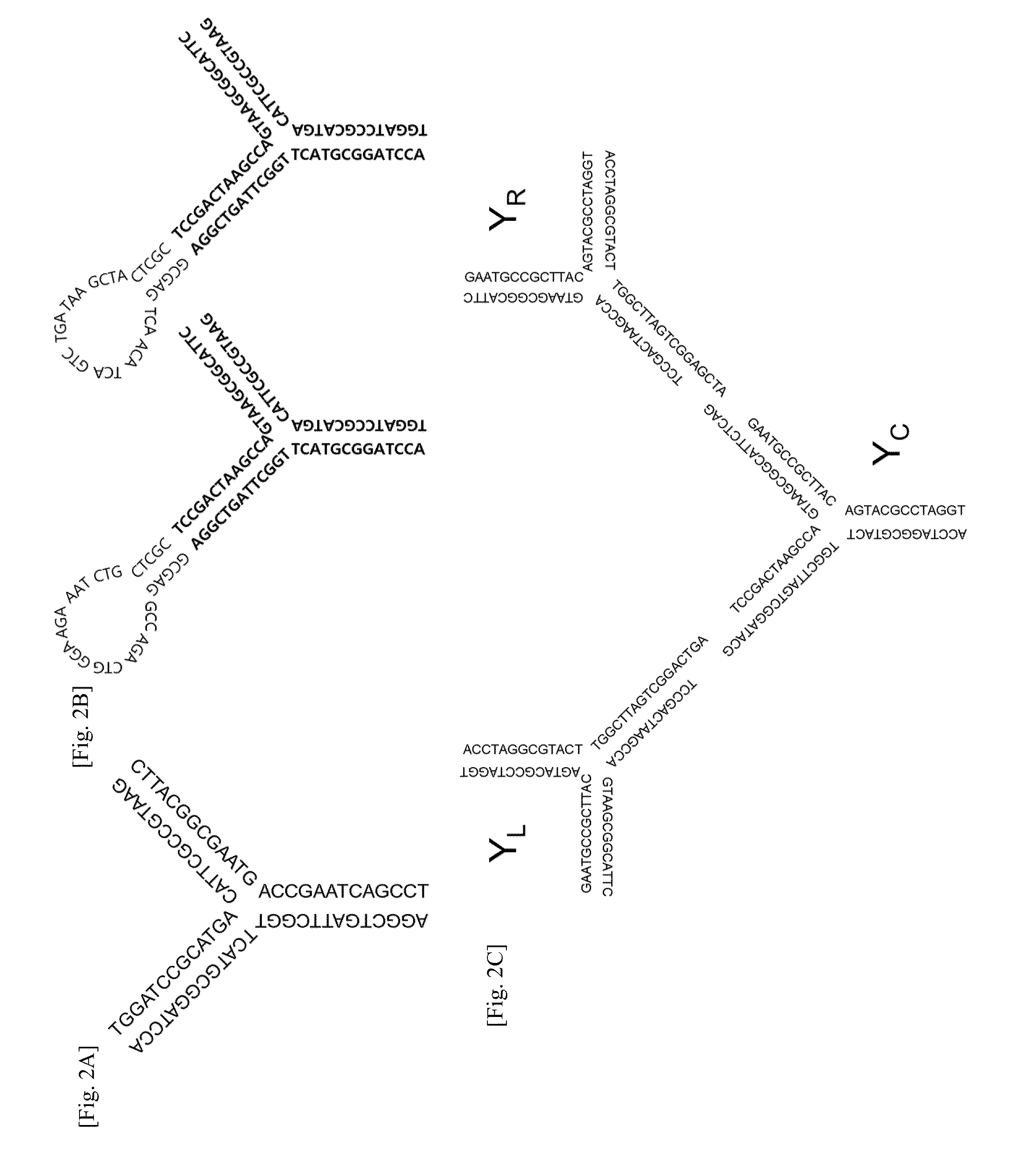
Nucleic acid nanostructure barcode probes
Provided herein are, inter alia, barcode probes comprised of transiently or stably fhiorescently labeled nucleic acid nanostructures that are fully addressable and able to be read using standard fluorescent microscope and methods of use thereof including methods of use as detectable labels for probes.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method and a device for monitoring nucleic acid amplification reactions
InactiveUS6310354B1Continuous measurementSimply performedBioreactor/fermenter combinationsBiological substance pretreatmentsCuvetteMicroparticle
A method for quantitatively measuring nucleic acid amplification reactions, especially the polymerase chain reaction, employing microparticles as hybridization solid phase, a probe sequence labeled with a fluorescent label and a fluorescence detection system which is based on two-photon fluorescence excitation, contacting all the amplification reaction components and the solid phase simultaneously in a closed cuvette, performing the amplification reactions in the same cuvette, focusing a two-photon exciting laser beam into the cuvette during the amplification cycles and measuring the fluorescence signal emitted by the microparticles from one particle at a time when they randomly float through the focal volume of the laser beam. The features of this invention allow a method and device for performing a fast quantitative nucleic acid amplification assay of single or multiple target sequences in a very small closed sample volume.
Owner:SOINI ERKKI
Hybridization chain reaction amplification for in situ imaging
ActiveUS20060228733A1Rapidly amplifyLow backgroundSugar derivativesMicrobiological testing/measurementAnalyteNucleic Acid Probes
The present invention relates to the use of fluorescently labeled nucleic acid probes to identify and image analytes in a biological sample. In the preferred embodiments, a probe is provided that comprises a target region able to specifically bind an analyte of interest and an initiator region that is able to initiate polymerization of nucleic acid monomers. After contacting a sample with the probe, labeled monomers are provided that form a tethered polymer. Triggered probes and self-quenching monomers can be used to provide active background suppression.
Owner:CALIFORNIA INST OF TECH
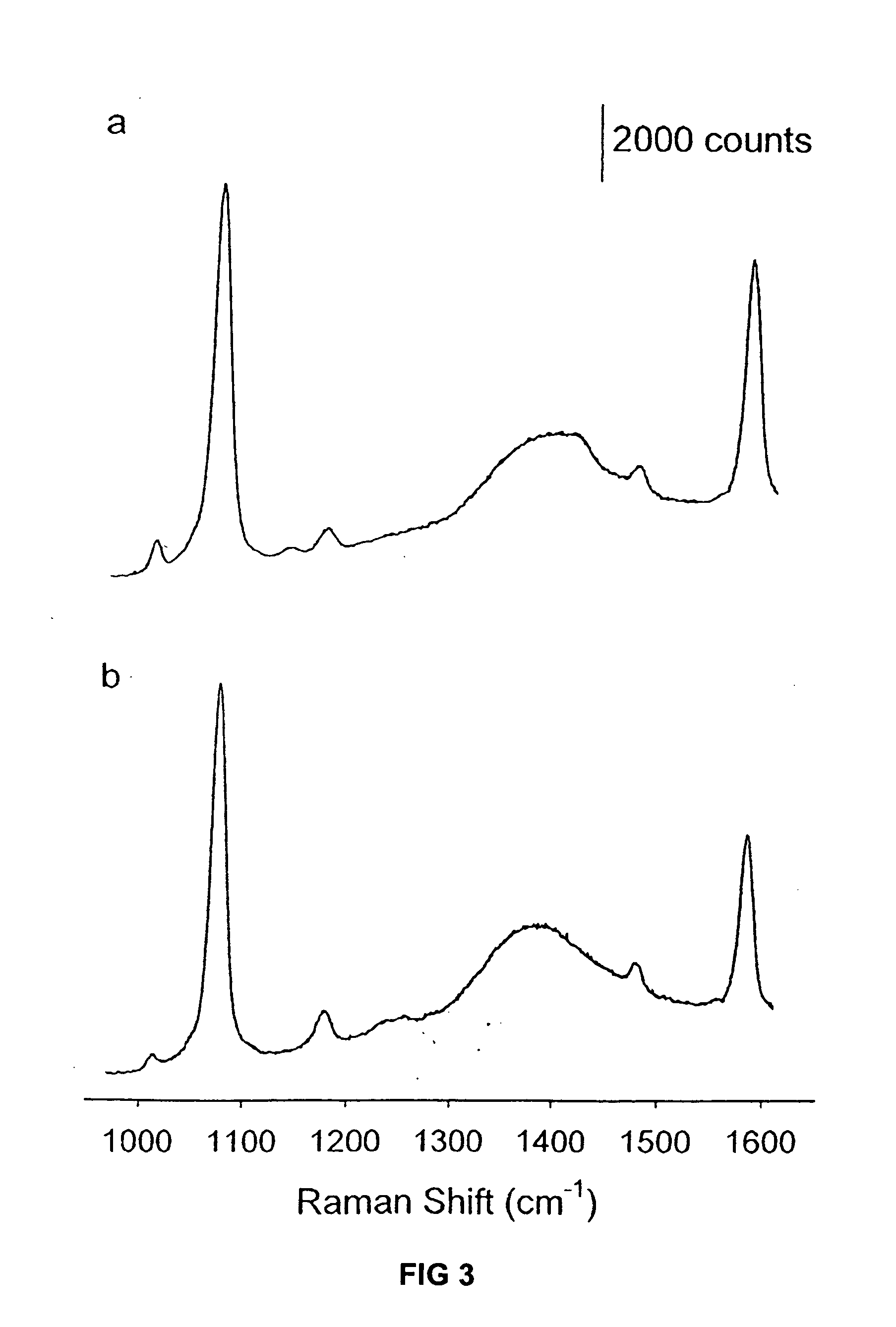
Raman-active reagents and the use thereof
InactiveUS20050089901A1Minimizes separationMaximizing numberHybrid immunoglobulinsSugar derivativesAntigenBiological materials
The present invention provides a new class of Raman-active reagents for use in biological and other applications, as well as methods and kits for their use and manufacture. Each reagent includes a Raman-active reporter molecule, a binding molecule, and a surface enhancing particle capable of causing surface enhanced Raman scattering (SERS). The Raman-active reporter molecule and the binding molecule are affixed to the particle to give both a strong SERS signal and to provide biological functionality, i.e. antigen or drug recognition. The Raman-active reagents can function as an alternative to fluorescence-labeled reagents, with advantages in detection including signal stability, sensitivity, and the ability to simultaneously detect several biological materials. The Raman-active reagents also have a wide range of applications, especially in clinical fields (e.g., immunoassays, imaging, and drug screening).
Owner:PORTER MARC D
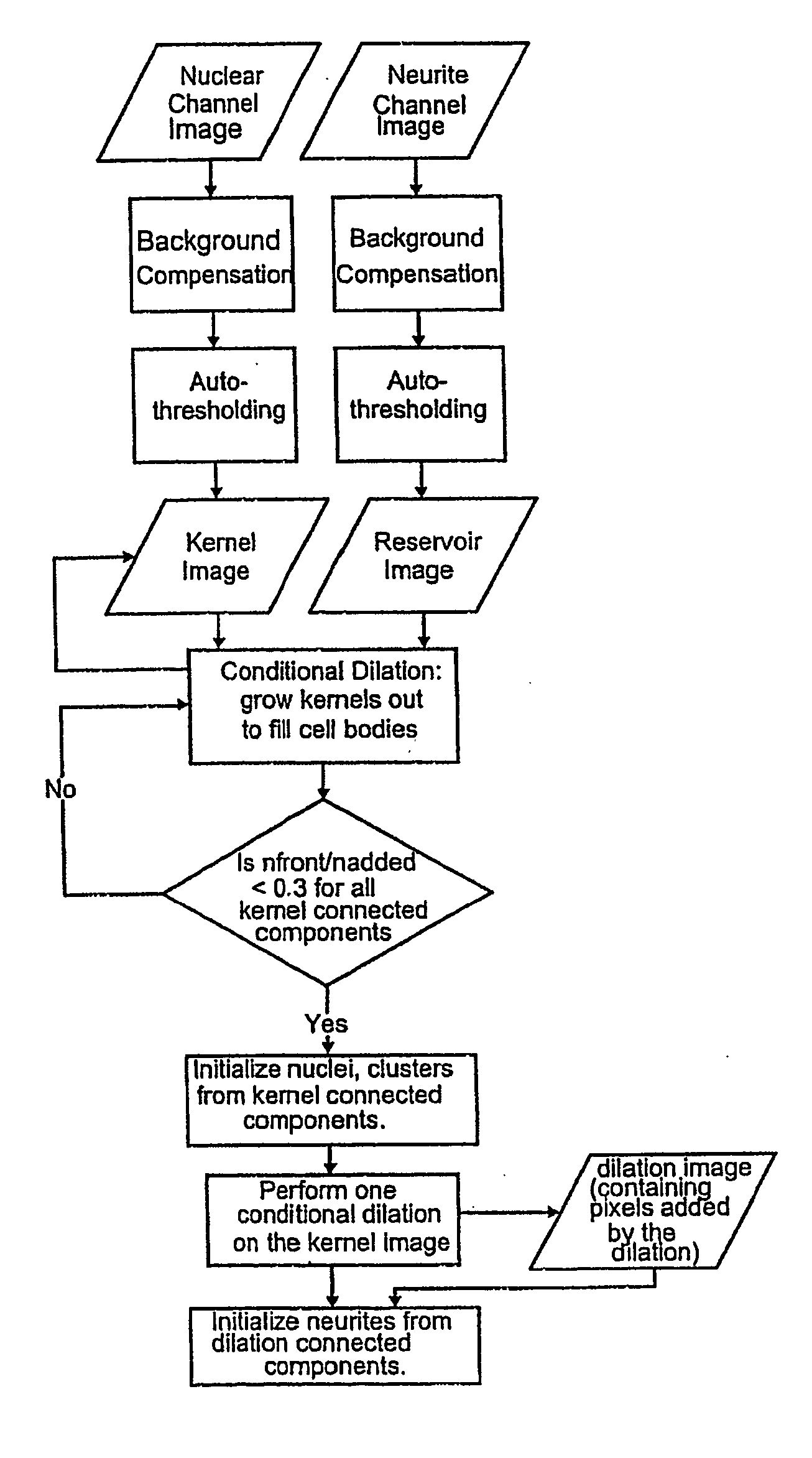
System for cell-based screening
The present invention provides systems, methods, screens, reagents and kits for optical system analysis of cells to rapidly determine the distribution, environment, or activity of fluorescently labeled reporter molecules in cells for the purpose of screening large numbers of compounds for those that specifically affect neurite outgrowth.
Owner:CELLOMICS
Method and an apparatus for localization of single dye molecules in the fluorescent microscopy
ActiveUS20090237501A1Simple processOvercome limitationsMaterial analysis by optical meansSignalling system detailsFluorescence microscopeSpatial direction
A method and apparatus are provided for obtaining a sub-resolution spatial information of a sample labeled with at least one type fluorescent label. The sub-resolution spatial information has localization information about the positions of fluorescent molecules of the at least one type fluorescent label in at least one spatial direction. The method acquires localization image data by employing fluorescence localization microscopy. The acquired localization image data is processed to obtain the localization information about the positions of fluorescent molecules of the at least one type fluorescent label in at least one spatial direction. The step of processing includes determining in each of the detected images of the series the positions of the barycenters of the detected fluorescence emission distributions from the single fluorescent molecules of the one or more fluorescent labels in at least one spatial direction.
Owner:UNIVERSITY OF HEIDELBERG
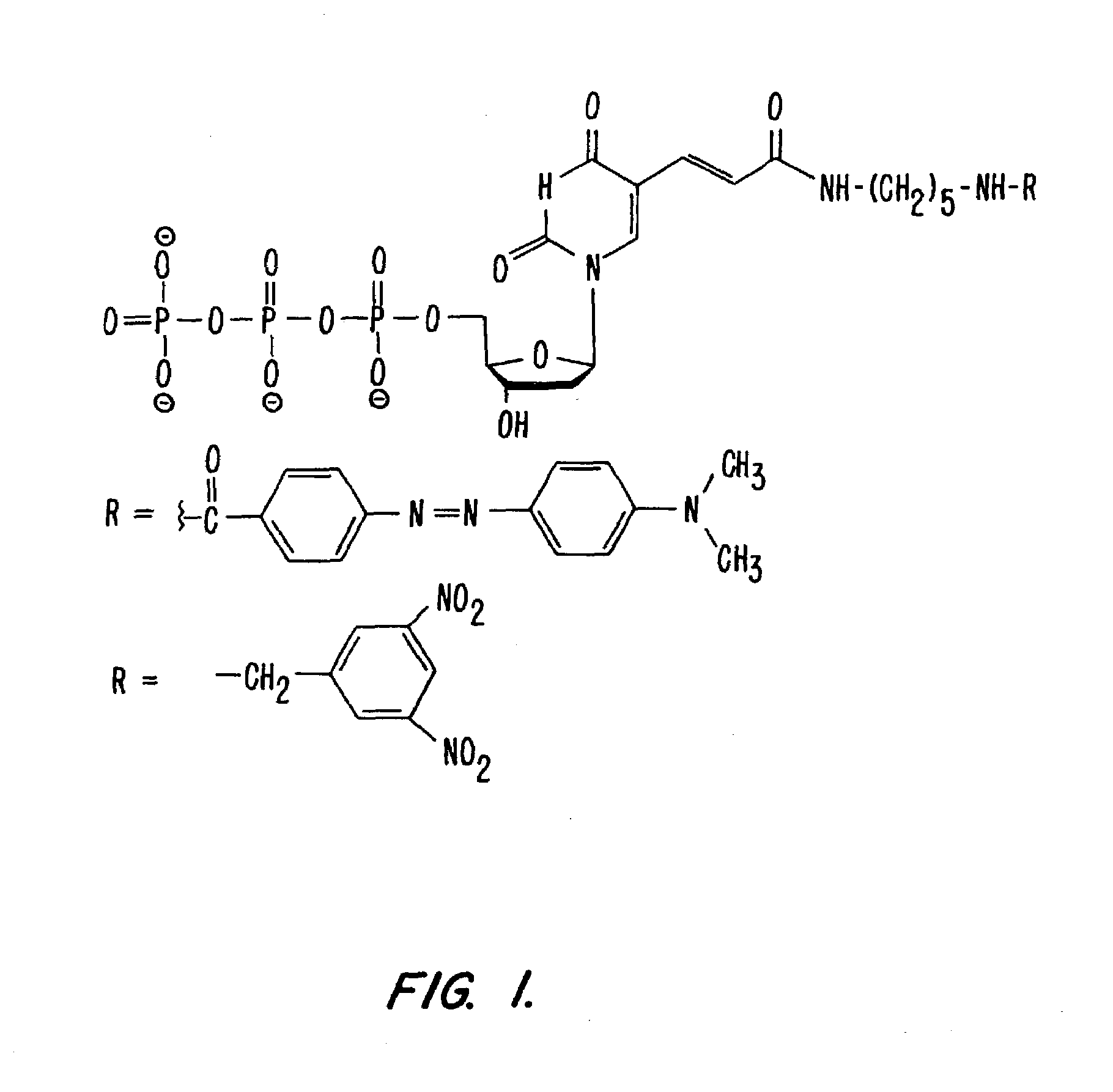
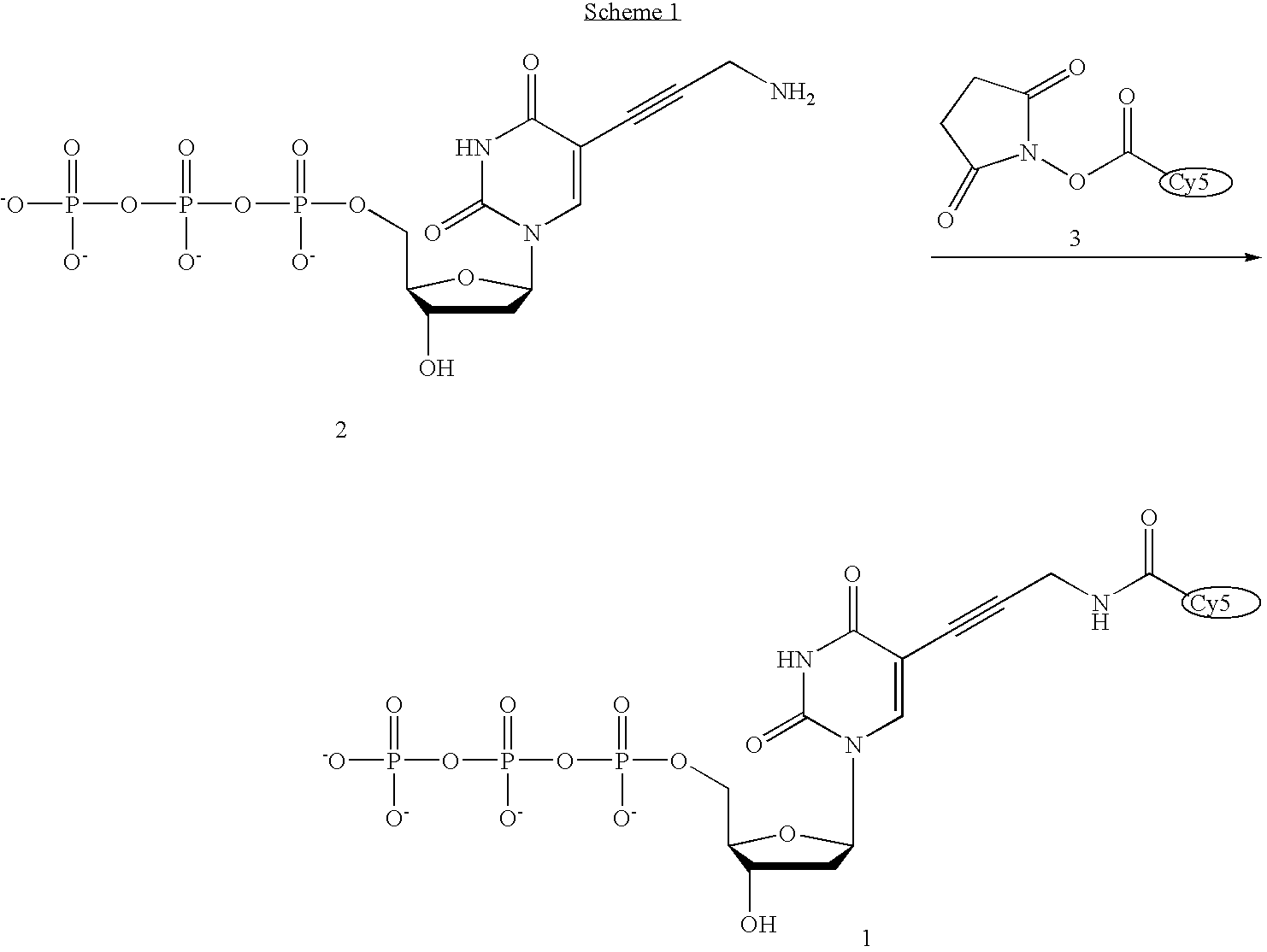
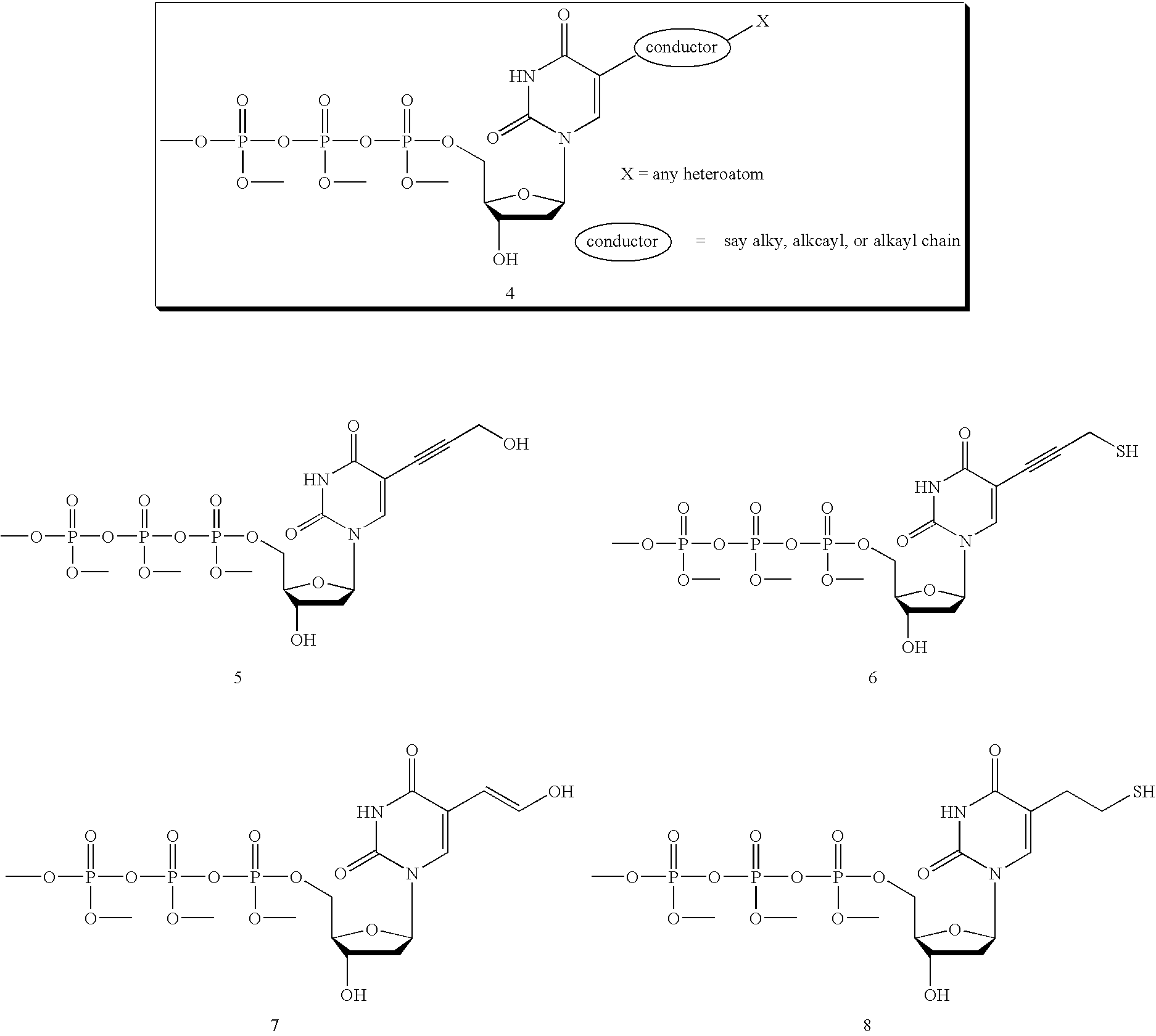
Fluorescently labeled nucleoside triphosphates and analogs thereof for sequencing nucleic acids
InactiveUS20050170367A1Reducing background fluorescenceEfficient excitationSugar derivativesMicrobiological testing/measurementNucleoside triphosphateFluorescent labelling
The invention provides methods for sequencing a nucleic acid, and particularly methods for synthesizing fluorescently labeled nucleoside triphosphates and related analogs for sequencing nucleic acids.
Owner:HELICOS BIOSCIENCES CORPORATION +1
Solid-state detector and optical system for microchip analyzers
InactiveUS6867420B2Easy to makeMaterial analysis by electric/magnetic meansSemiconductor/solid-state device manufacturingElectrophoresesOptical fluorescence
Owner:PALO ALTO RES CENT INC +1
Fluorescence Detection
InactiveUS20080265177A1Bioreactor/fermenter combinationsBiological substance pretreatmentsUltravioletLight emission
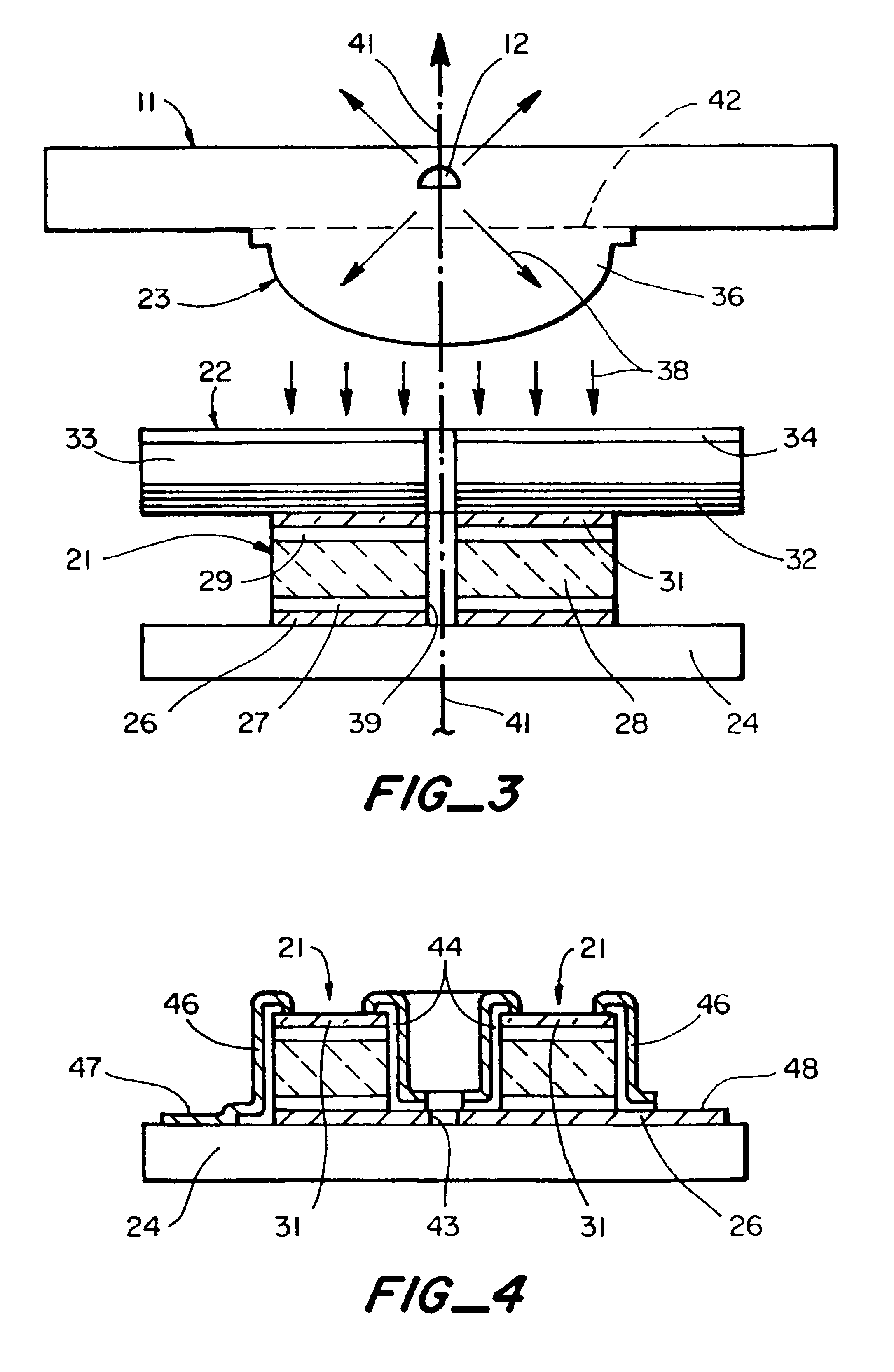
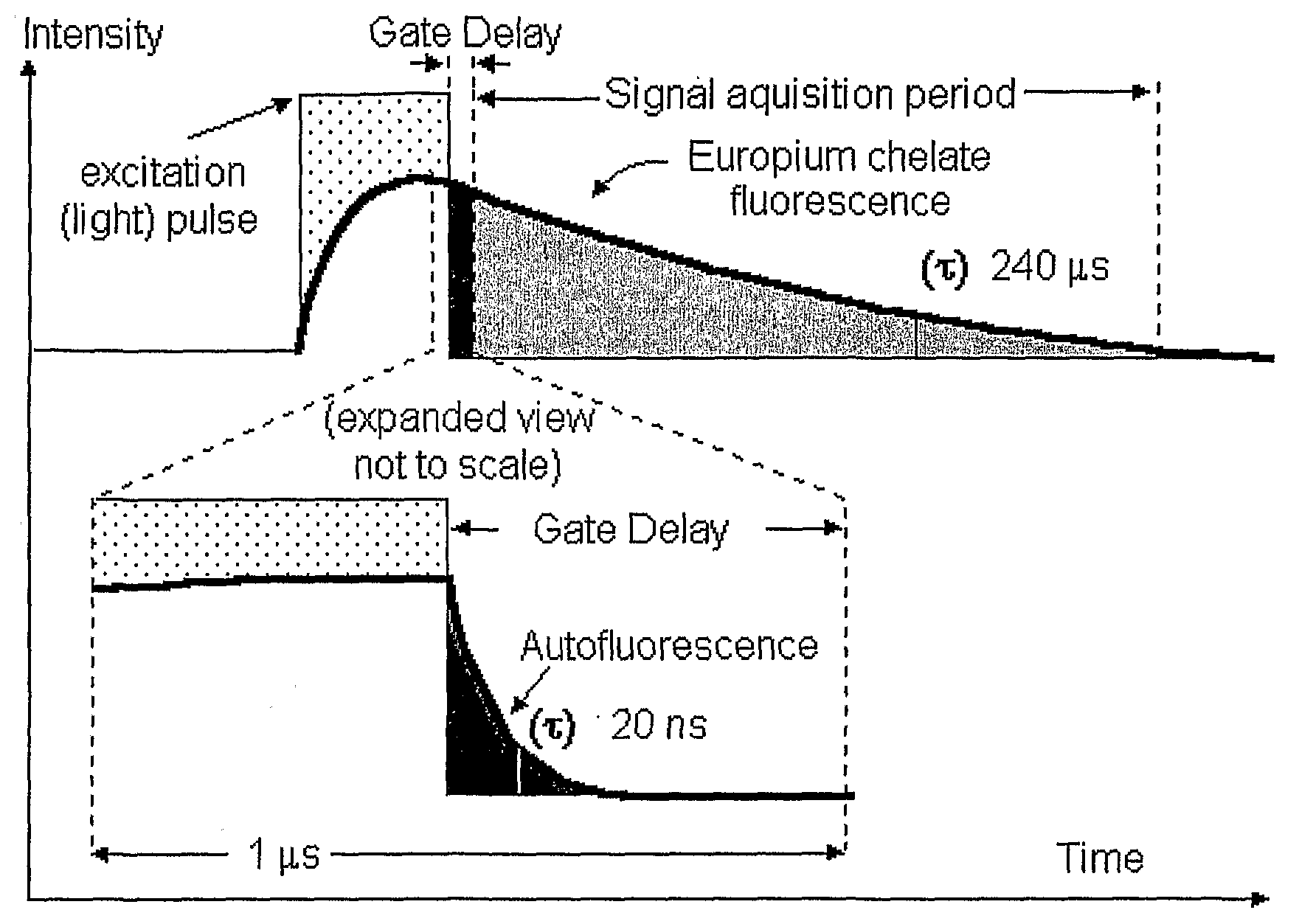
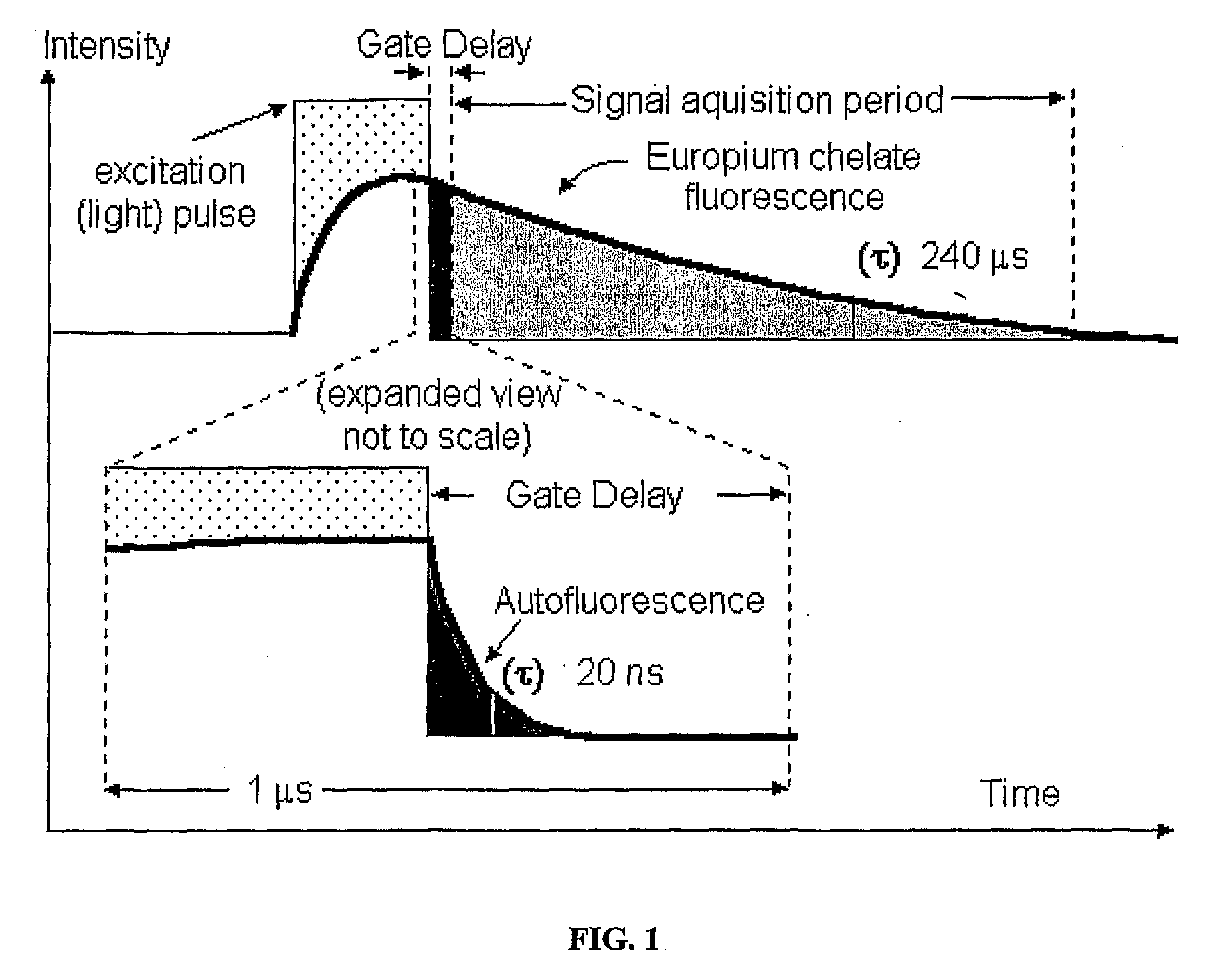
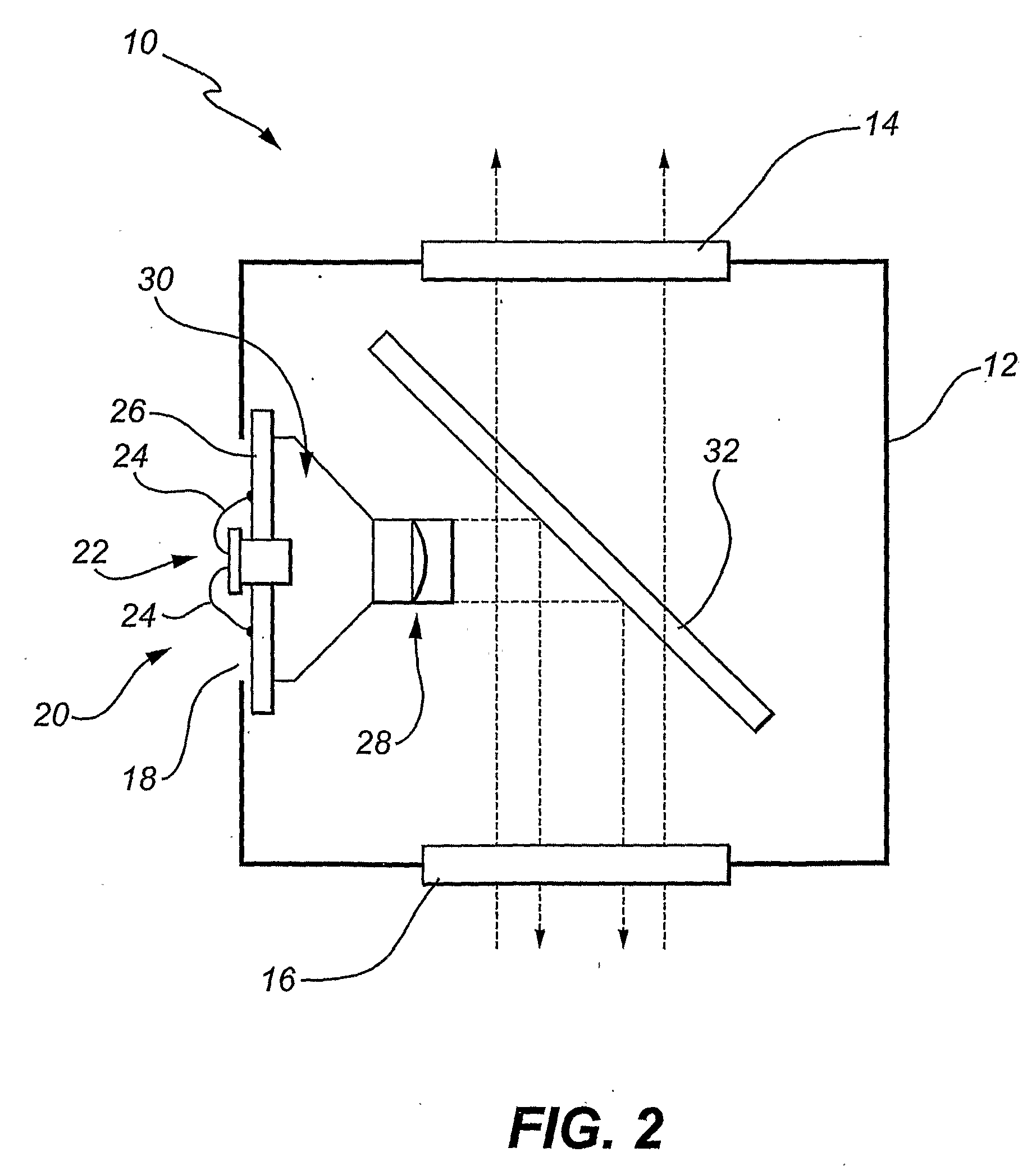
A fluorescence detection system comprises a light source (22), dichroic mirror (32), excitation port (16), emission port (14), and a detector. The light source (22) is, for example, a pulsed ultraviolet LED, with a light emission that decays sufficiently rapidly to permit gated detection of fluorescence from a fluorescently-labelled species, at a time when it is distinguishable from autofluorescence. The detector is, for example, an electron multiplying CCD, with high gain on-chip amplification. A circuit (26) may be used to control a repeating cycle of (i) generation of a 20-200 microsecond UV. pulse; (ii) a gate delay of 1-5 microseconds; and (iii) a 10-800 microsecond detection period. This allows time-resolved-fluorescence-microscopy with real time or near real time operation.
Owner:MACQUARIE UNIV
Methods and algorithms for cell enumeration in a low-cost cytometer
InactiveUS20070117158A1Simple designReduce operating costsBioreactor/fermenter combinationsImage analysisWhite blood cellCcd camera
The enumeration of cells in fluids by flow cytometry is widely used across many disciplines such as assessment of leukocyte subsets in different bodily fluids or of bacterial contamination in environmental samples, food products and bodily fluids. For many applications the cost, size and complexity of the instruments prevents wider use, for example, CD4 analysis in HIV monitoring in resource-poor countries. The novel device, methods and algorithms disclosed herein largely overcome these limitations. Briefly, all cells in a biological sample are fluorescently labeled, but only the target cells are also magnetically labeled. In addition, non-magnetically labeled cells are imaged for viability in a modified slide configuration. The labeled sample, in a chamber or cuvet, is placed between two wedge-shaped magnets to selectively move the magnetically labeled cells to the observation surface of the cuvet. An LED illuminates the cells and a CCD camera captures the images of the fluorescent light emitted by the target cells. Image analysis performed with a novel algorithm provides a count of the cells on the surface that can be related to the target cell concentration of the original sample. The compact cytometer system provides a rugged, affordable and easy-to-use technique, which can be used in remote locations.
Owner:UNIVERSITY OF TWENTE
Acoustic standing-wave enhancement of a fiber-optic salmonella biosensor
InactiveUS6391653B1Bioreactor/fermenter combinationsBiological substance pretreatmentsMicrosphereTest chamber
A fluorescent fiber-optic biosensor system using ultrasonic concentration of particles and cells for the detection of Salmonella typhimurium. A biosensor test chamber serves as an ultrasonic standing-wave cell that allows microspheres or cells to be concentrated in parallel layers or in a column along the axis of the cell. A fiber probe along the axis delivers laser excitation to fluorescent-labeled antibodies of Salmonella and collects the fluorescent signal. The Salmonella-antibody complexes are moved acoustically to the axis of the cell, increasing the fluorescent signal. Alternatively, the Salmonella-labelled antibody complexes attach to unlabeled antibodies that have been immobilized on the surface of polystyrene microspheres. This entire structure can be manipulated acoustically and the increase in the fluorescent signal, which can be an order of magnitude, indicates the presence of Salmonella.
Owner:BOARD OF GOVERNORS FOR HIGHER EDUCATION STATE OF RHODE ISLAND & PROVIDENCE PLANTATIONS
Single molecule spectroscopy for analysis of cell-free nucleic acid biomarkers
InactiveUS20120135874A1Quick analysisHinder acceptanceMicrobiological testing/measurementLibrary screeningFluorescent lightFluorescent labelling
The present invention relates, e.g., to a method for detecting a nucleic acid molecule of interest in a sample comprising cell-free nucleic acids, comprising fluorescently labeling the nucleic acid molecule of interest, by specifically binding a fluorescently labeled nanosensor or probe to the nucleic acid of interest, or by enzymatically incorporating a fluorescent probe or dye into the nucleic acid of interest, illuminating the fluorescently labeled nucleic acid molecule, causing it to emit fluorescent light, and measuring the level of fluorescence by single molecule spectroscopy, wherein the detection of a fluorescent signal is indicative of the presence of the nucleic acid of interest in the sample.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Transilluminator with ultraviolet light emitting diode array
InactiveUS20060237658A1UniformityImprove uniformityColor/spectral properties measurementsPhotometry using electric radiation detectorsFluorescencePhysics
A method and apparatus for genomic or proteomic research to visualize fluorescent labeled DNA, RNA or protein samples that have been separated for documentation and analysis. The apparatus includes a novel radiation source for uniformly irradiating the samples which comprises an array of UV LEDS. In one form of the invention the apparatus also includes a first conversion plate that is carried by the housing at a location intermediate the radiation source and the sample supporting platform for converting the radiation emitted from the source to radiation at a second wavelength.
Owner:WALUSZKO ALEX
System for cell-based screening
InactiveUS6986993B1Increase contentBioreactor/fermenter combinationsBiological substance pretreatmentsSystems analysisCell based
The present invention provides systems, methods, screens, reagents and kits for optical system analysis of cells to rapidly determine the distribution, environment, or activity of fluorescently labeled reporter molecules in cells for the purpose of screening large numbers of compounds for those that specifically affect neurite outgrowth.
Owner:CELLOMICS
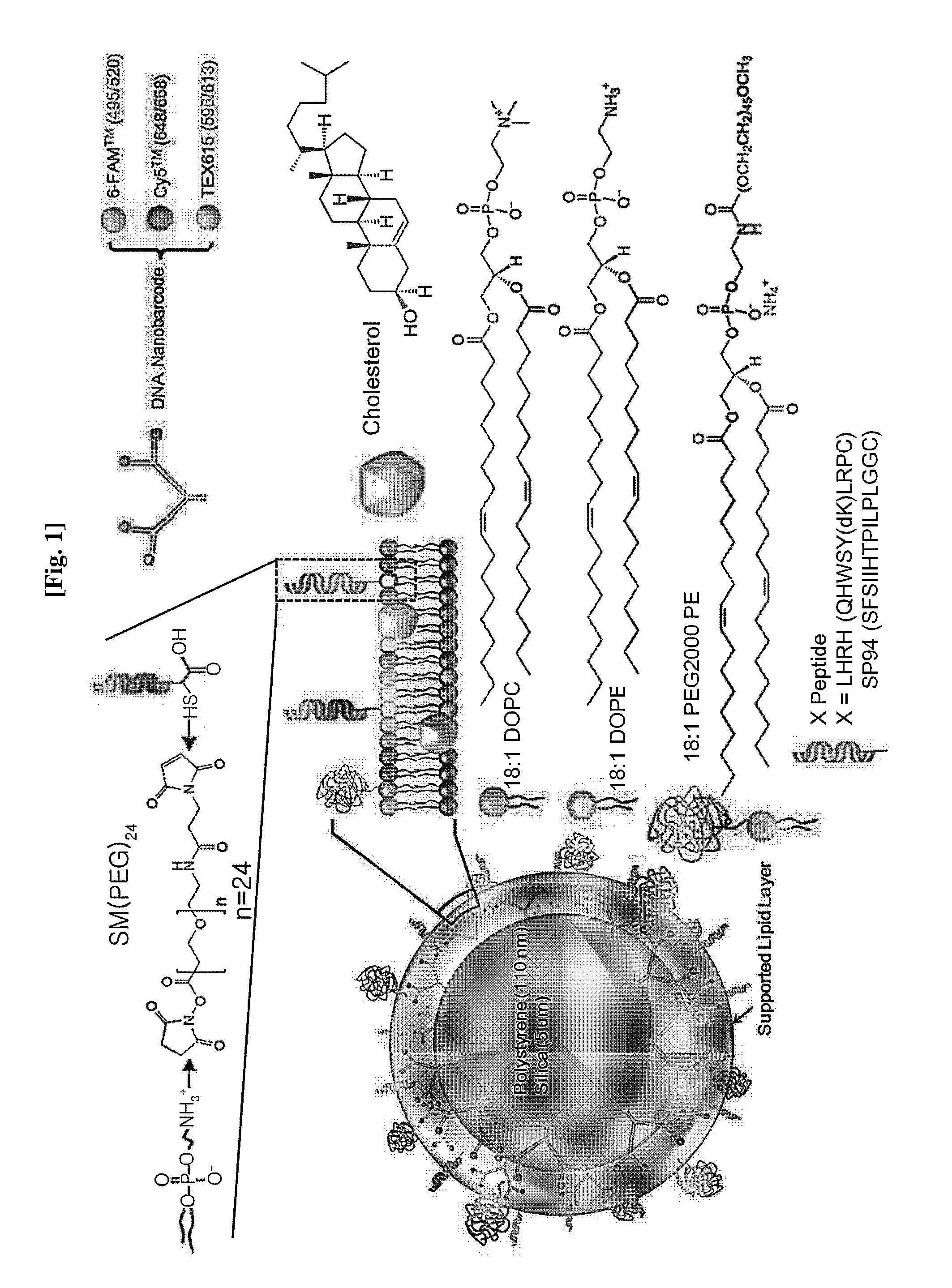
Fusion NANO liposome-fluorescence labeled nucleic acid for in vivo application, uses thereof and preparation method thereof
InactiveUS20150211056A1Well formedUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsNucleic acid structureCell membrane
The present disclosure relates to a fusion nano liposome-fluorescence labeled nucleic acid in which a bead having a surface binding with a branch-shaped nucleic acid structure labeled with a fluorophore or a branch-shaped nucleic acid structure having a hairpin loop end is included in an inside of a liposome, and a diagnosis application thereof. The fusion nano liposome-fluorescence labeled nucleic acid, or fusion nano liposome-fluorescence labeled hairpin loop structured nucleic acid may sense an external or internal signal, and high-sensitive diagnosis is possible even when mRNA and miRNA which is present at a low concentration in cells being targeted. Further, various target materials expressed inside and outside of a cell membrane may be targeted, and thus even a type of cancer which is hard to diagnose such as triple negative breast cancer also be flexibly diagnosed. Further, using various fluorophores, multiple cancer may be diagnosed at the same time.
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
Fluorescence proximity assay
InactiveUS20030059850A1Easy to operateEasy to useMicrobiological testing/measurementBiological testingColloidFluorophore
The present invention provides binding assays, referred to here as fluorescence proximity assays or FPA. The inventions detect binding of target molecules in a sample to a molecular probe or probes that specifically bind or hybridize to those molecules. In particular, the molecular probes are immobilized to a bead or particle, such as colloidal gold, the reflects fluorescent energy from a fluorophore. The derivatized beads are contacted to a sample of fluorescently labeled target molecules, and binding of the target is indicated by an increase in the fluorescent signal. Kits are also provided that contain materials and reagents to performing a fluorescence proximity assay.
Owner:STANLEY MEDICAL RES HLDG
Fluorine-boron fluorescent dye as well as preparation method and application thereof
InactiveCN103865290ANarrow absorbencyNarrow emission peakAzo dyesGroup 3/13 element organic compoundsQuantum yieldHalogen
The invention discloses a fluorine-boron fluorescent dye as well as a preparation method and application thereof, wherein the structure of the fluorine-boron fluorescent dye is shown as a formula (I) or a formula (II), in the formula (I) and the formula (II), R1 is H or halogen; R2 is CN; R3, R4, R5 and R6 are independently selected from H, halogen, C1-C6 alkyl or C1-C6 alkoxy; V, W, X, Y and Z are independently CH or N, and when V, W, X, Y or Z is N, N has no substituent group. According to the fluorine-boron fluorescent dye and the preparation method thereof, the maximal fluorescence emission wavelength of the fluorine-boron fluorescent dye is 518-600nm, and the fluorine-boron fluorescent dye also has excellent fluorescence quantum yield and Stokes shift, which shows that the fluorine-boron fluorescent dye has good application prospect in the bioanalysis fields of fluorescence labeling, bioimaging and so on; meanwhile, the preparation method is simple in steps, and raw materials can be obtained easily.
Owner:ANHUI NORMAL UNIV
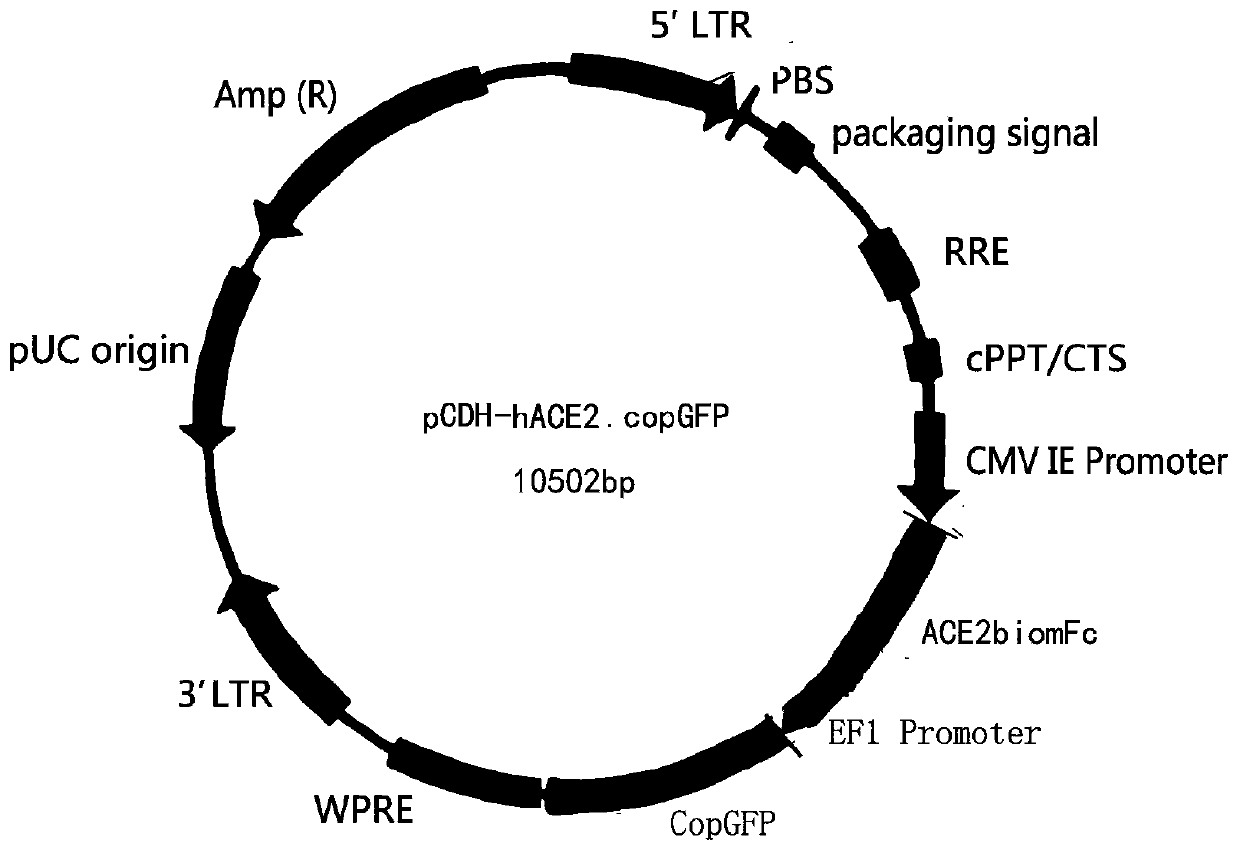
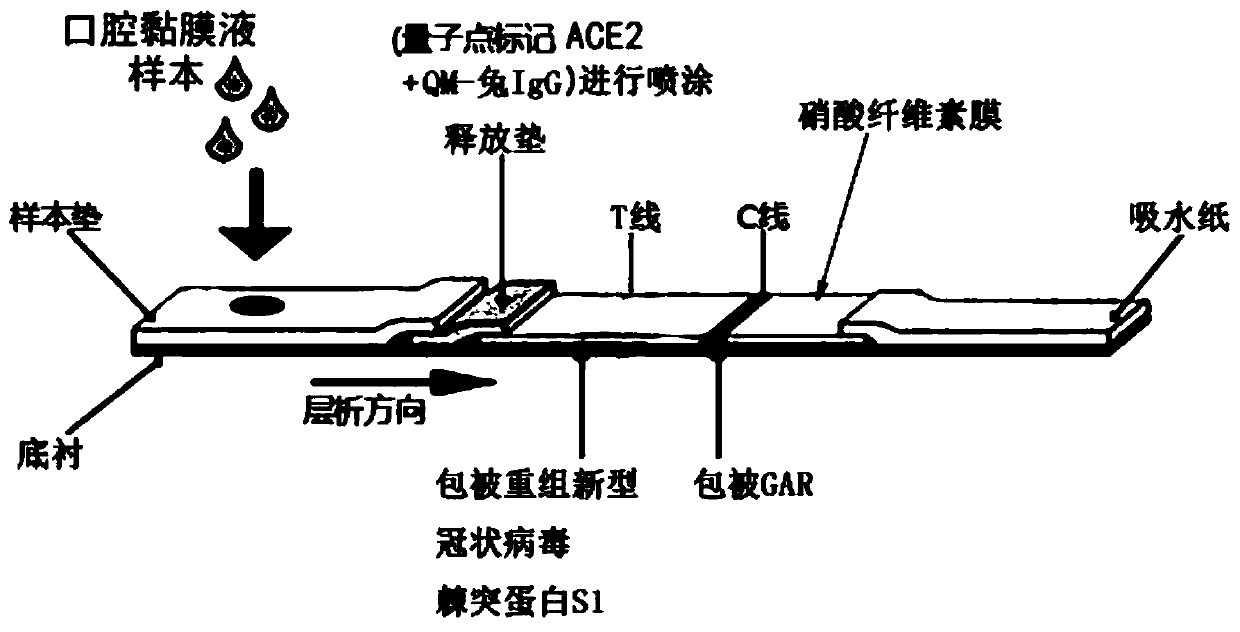
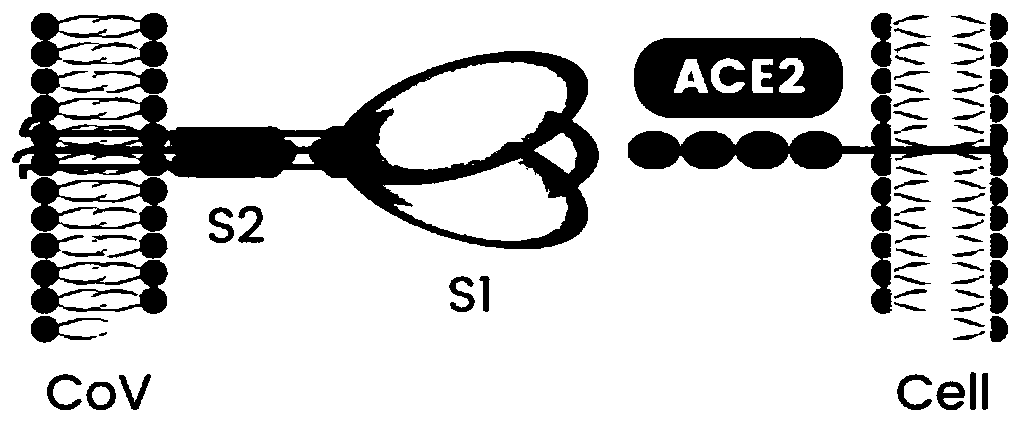
Coronavirus rapid detection kit based on S protein ligand and ACE2 receptor competitive chromatography
ActiveCN111273016AImmunochromatographic fastEasy immunochromatographyCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsReceptorBlood plasma
Owner:浙江诺迦生物科技有限公司 +1
Multicolor chromogenic detection of biomarkers
InactiveUS20080299555A1Facilitates chromogenic detection of signalThe result is accurateMicrobiological testing/measurementBiological testingHistocytochemistryChemiluminescence
The present invention provides compositions, kits, assembles of articles and methodology for detecting multiple target molecules in a sample, such as in a tissue sample. In particular, site-specific deposition of elemental metal is used in conjunction with other means of detection, such as other chromogenic, radioactive, chemiluminescent and fluorescent labeling, to simultaneously detect multiple targets, such a gene, a protein, and a chromosome, in a biological sample. More particularly the multiple targets may be labeled with the specifically deposited metal and other chromogenic labels to allow chromogenic immunohistochemical (IHC) detection in situ by using bright field light microscope.
Owner:VENTANA MEDICAL SYST INC
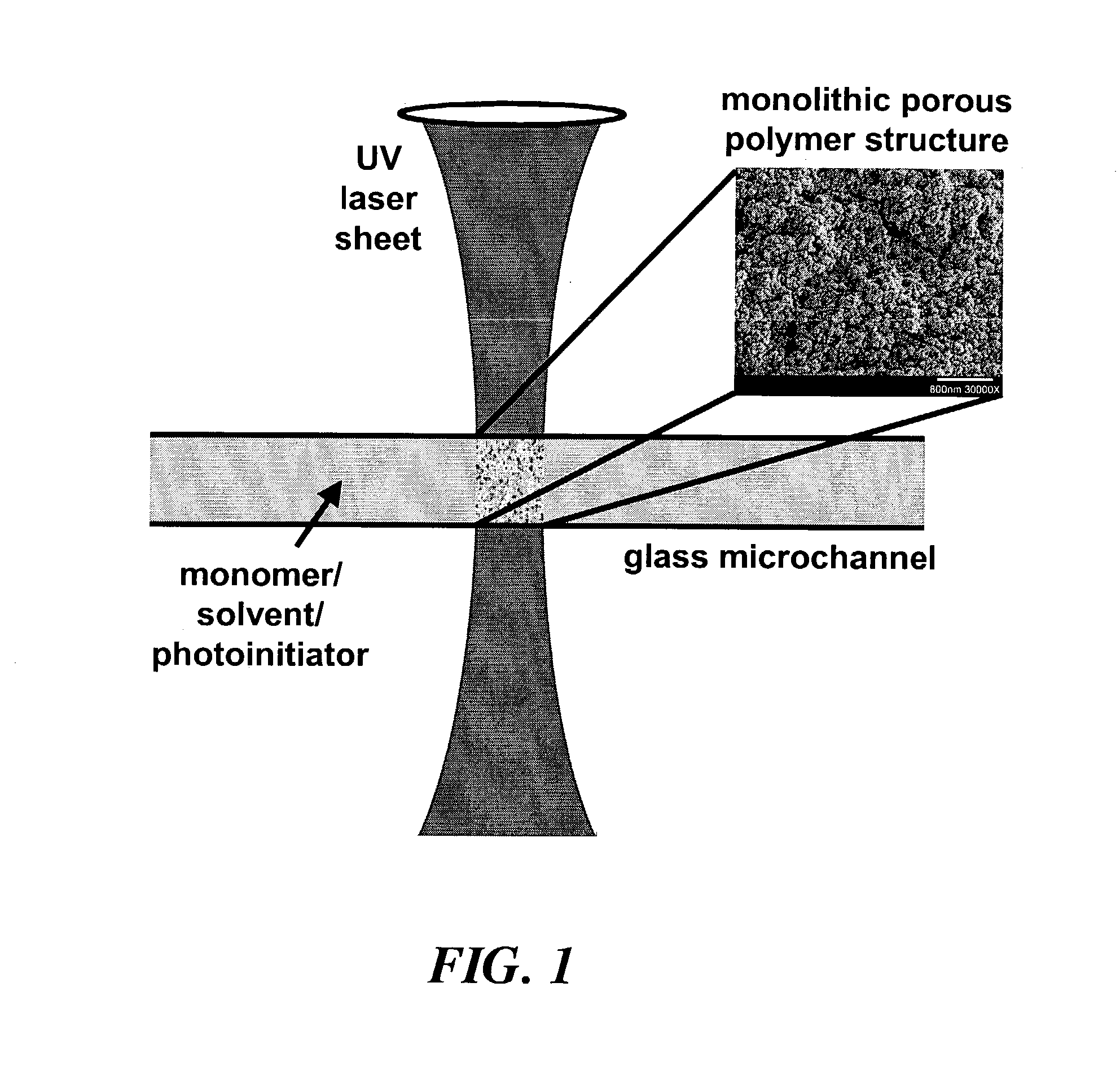
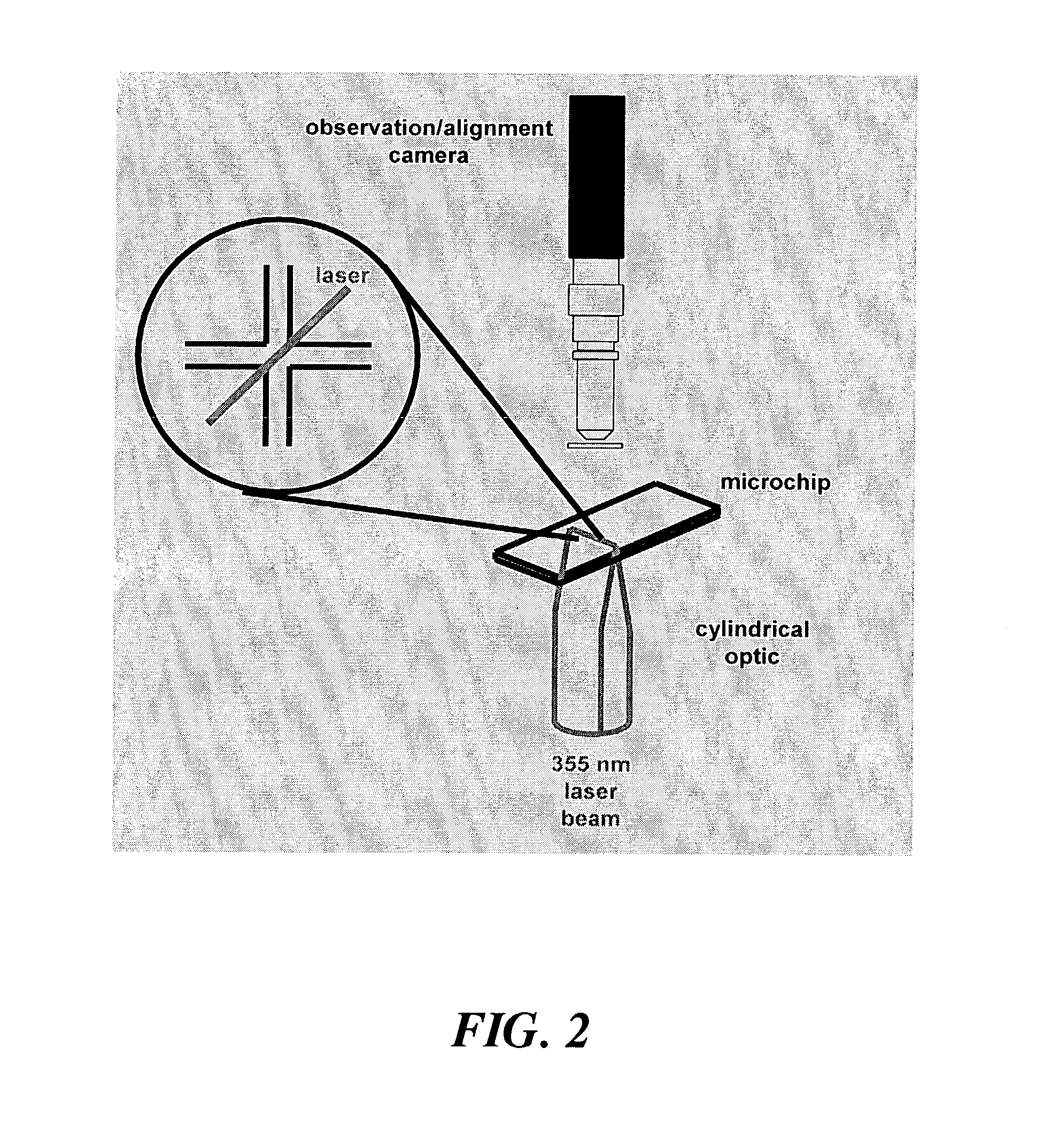
Dialysis on microchips using thin porous polymer membranes
ActiveUS7264723B2Minimize handlingComplicated operationSolvent extractionTransportation and packagingDialysis membranesMeth-
Laser-induced phase-separation polymerization of a porous acrylate polymer is used for in-situ fabrication of dialysis membranes inside glass microchannels. A shaped 355 nm laser beam is used to produce a porous polymer membrane with a thickness of about 15 μm, which bonds to the glass microchannel and form a semi-permeable membrane. Differential permeation through a membrane formed with pentaerythritol triacrylate was observed and quantified by comparing the response of the membrane to fluorescein and fluorescently tagging 200 nm latex microspheres. Differential permeation was observed and quantified by comparing the response to rhodamine 560 and lactalbumin protein in a membrane formed with SPE-methylene bisacrylamide. The porous membranes illustrate the capability for the present technique to integrate sample cleanup into chip-based analysis systems.
Owner:SANDIA NAT LAB
Fluorescence immune chromatography test paper and preparing method and application thereof
InactiveCN101526534AHigh sensitivityOvercome the easy-to-quench defectBiological testingFiberAntigen
The invention relates to fluorescence immune chromatography test paper which is formed by mutually and sequentially overlapping a sample pad, a combination pad, an antibody carrying film and water absorbing paper on a lining board with adhesive, wherein the combination pad is coated with a fluorescence material antibody 1 composite, a fluorescence-marked material is fluorescein granules or fluorescence material converted by rare earth, the antibody carrying film is a cellulose nitrate film or a nylon film and is respectively connected with an antibody 2 and the T line and the C line of a secondary antibody, the fluorescence material is fluorescein granules or the fluorescence granules converted by the rare earth, and the fluorescein granules are selected from isosulfocyanic acid fluorescein or tetramethyl isosulfocyanic acid rhodamine or tetraethyl rhodamine, and the surface of the fluorescence-marked material is aminated and combined with the antibody by cross-linking reaction. The fluorescence quantitative measuring system is used for detecting the fluorescence strength of the areas of the T line and the C line, and the quantitative detection is completed by the standard curve of the antigen concentration. The invention is suitable for the immune detection of tumor marked objects of urinal fiber-connected protein, and the like.
Owner:沈鹤柏
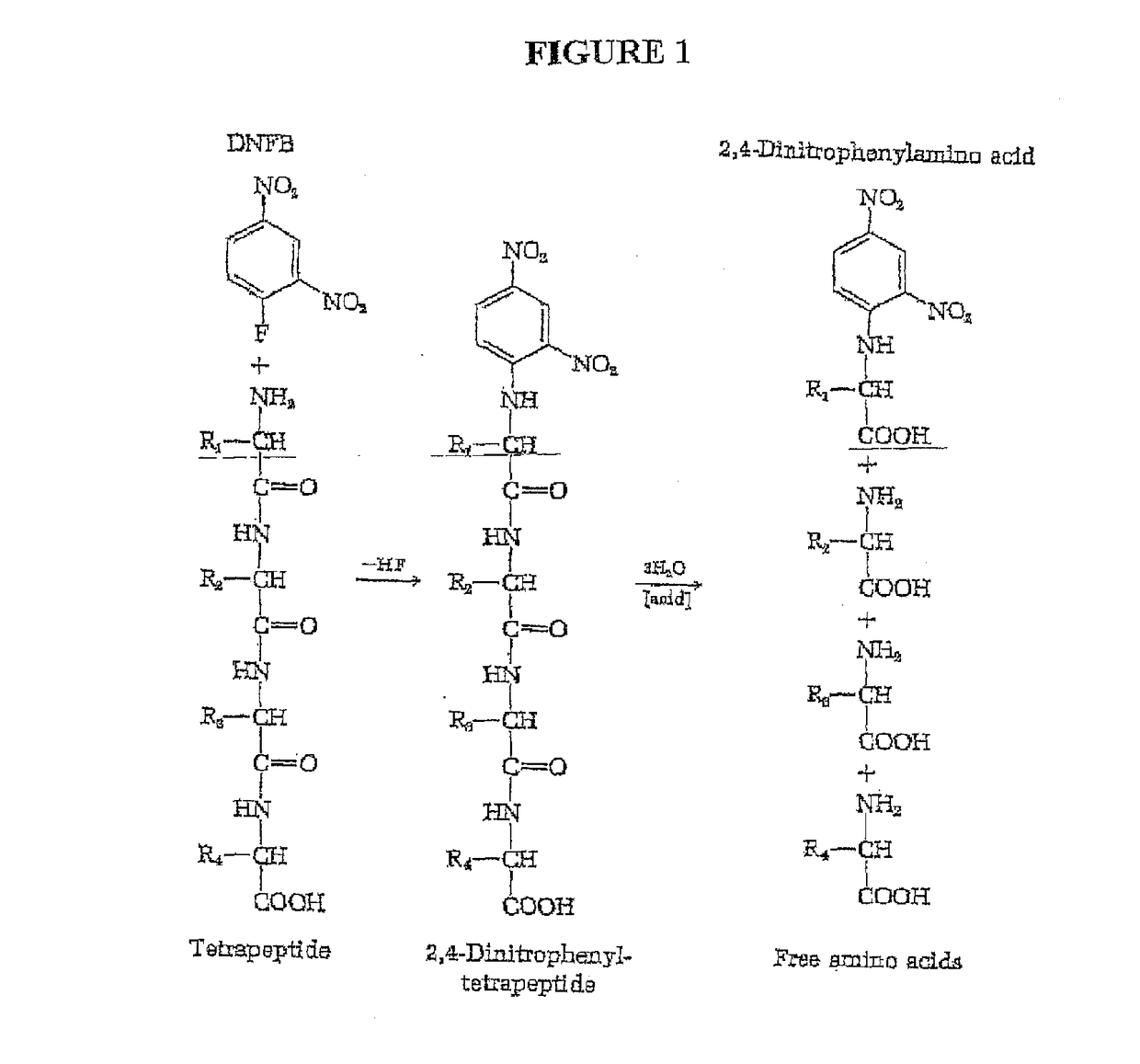
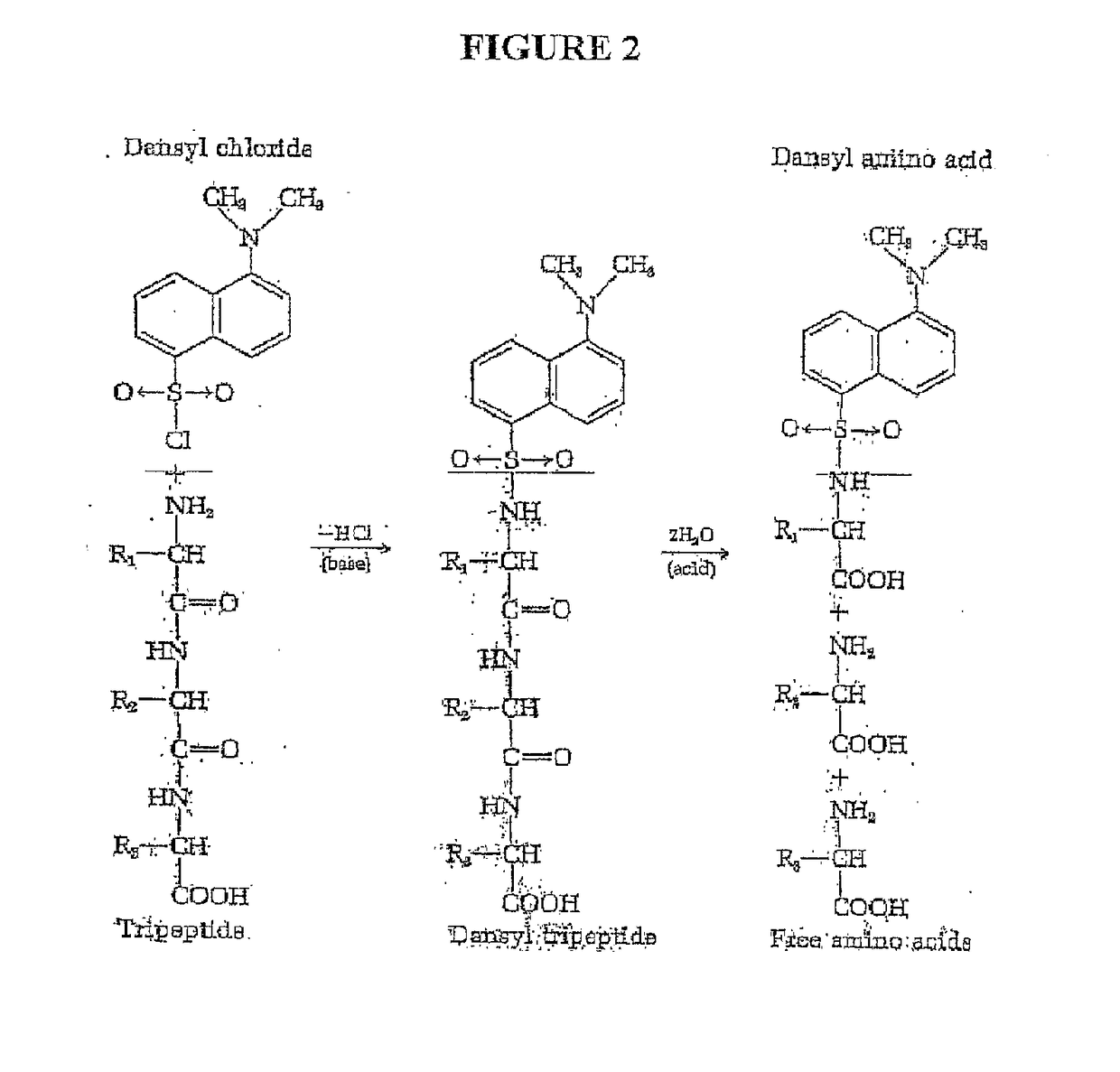
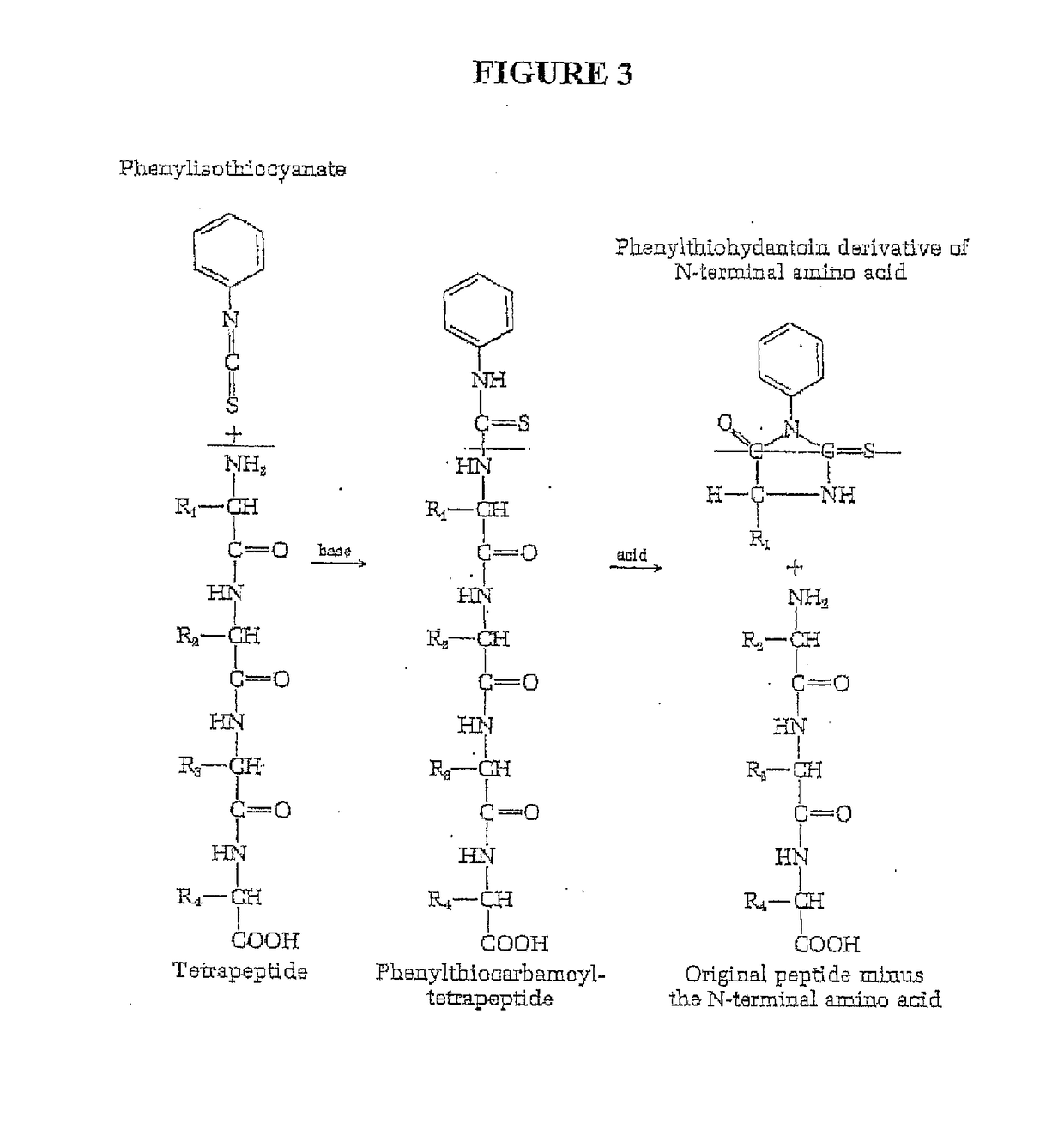
Single molecule peptide sequencing
The present invention relates to the field of identifying proteins and peptides, and more specifically large-scale sequencing of single peptides in a mixture of diverse peptides at the single molecule level. The present invention also relates to methods for identifying amino acids in peptides, including peptides comprising unnatural amino acids. In one embodiment, the present invention contemplates labeling the N-terminal amino acid with a first label and labeling an internal amino acid with a second label. In some embodiments, the labels are fluorescent labels. In other embodiments, the internal amino acid is Lysine. In other embodiments, amino acids in peptides are identified based on the fluorescent signature for each peptide at the single molecule level.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method of nucleic acid sequence determination
ActiveUS20170314072A1Unable to formatMicrobiological testing/measurementTransferasesNucleotideFluorescence
Provided are sequencing-by-binding methods of detecting cognate nucleotides using a crippled DNA polymerizing enzyme that possesses the ability to bind the next correct nucleotide downstream of a primer in a template-dependent fashion, but does not possess the activity needed to promote phosphodiester bond formation. Use of the crippled DNA polymerase permits interrogation of one nucleotide at a time, without incorporation of any nucleotide. Labeled nucleotides, such as fluorescently labeled nucleotides, can be used in conjunction with the crippled DNA polymerase to establish cognate nucleotide identity in a rapid manner.
Owner:PACIFIC BIOSCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com