Endoscope system for fluorescent observation
a fluorescent observation and endoscope technology, applied in the field of endoscopes, can solve the problems of remarkably adverse factors, inapplicability of conventional techniques focusing on morphologic changes in cells for determining the presence of cancer, and the fact that the tissues to be observed are within a living body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
first embodiment
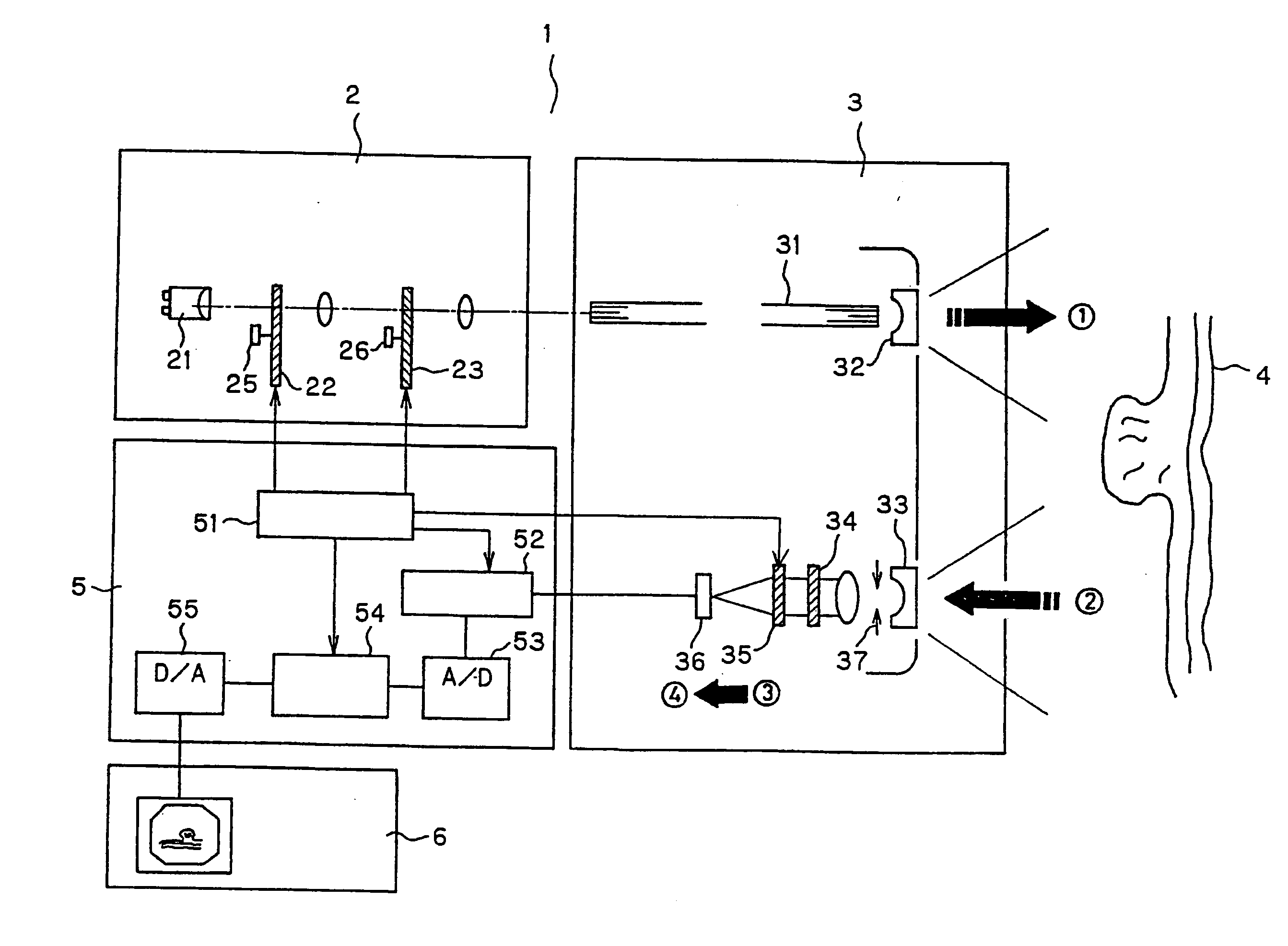
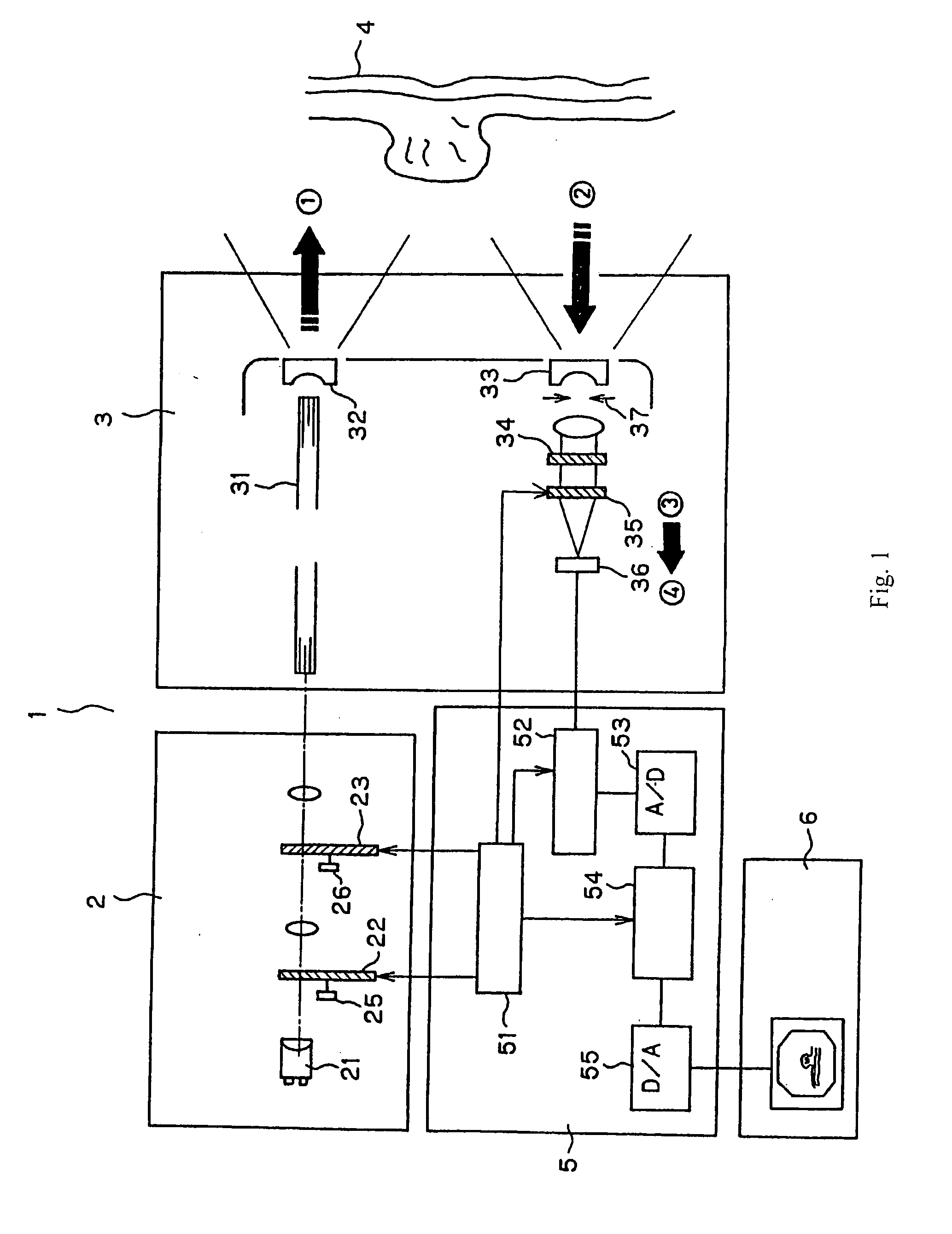
FIG. 1 shows the entire structure of the endoscope system according to the present invention. In FIG. 1, an endoscope system 1 is formed of a light source system 2, an endoscope 3, a processor 5, and a monitor 6. This embodiment is characterized by having a structure that separates and detects plural fluorescent wavelengths within the endoscope tip.
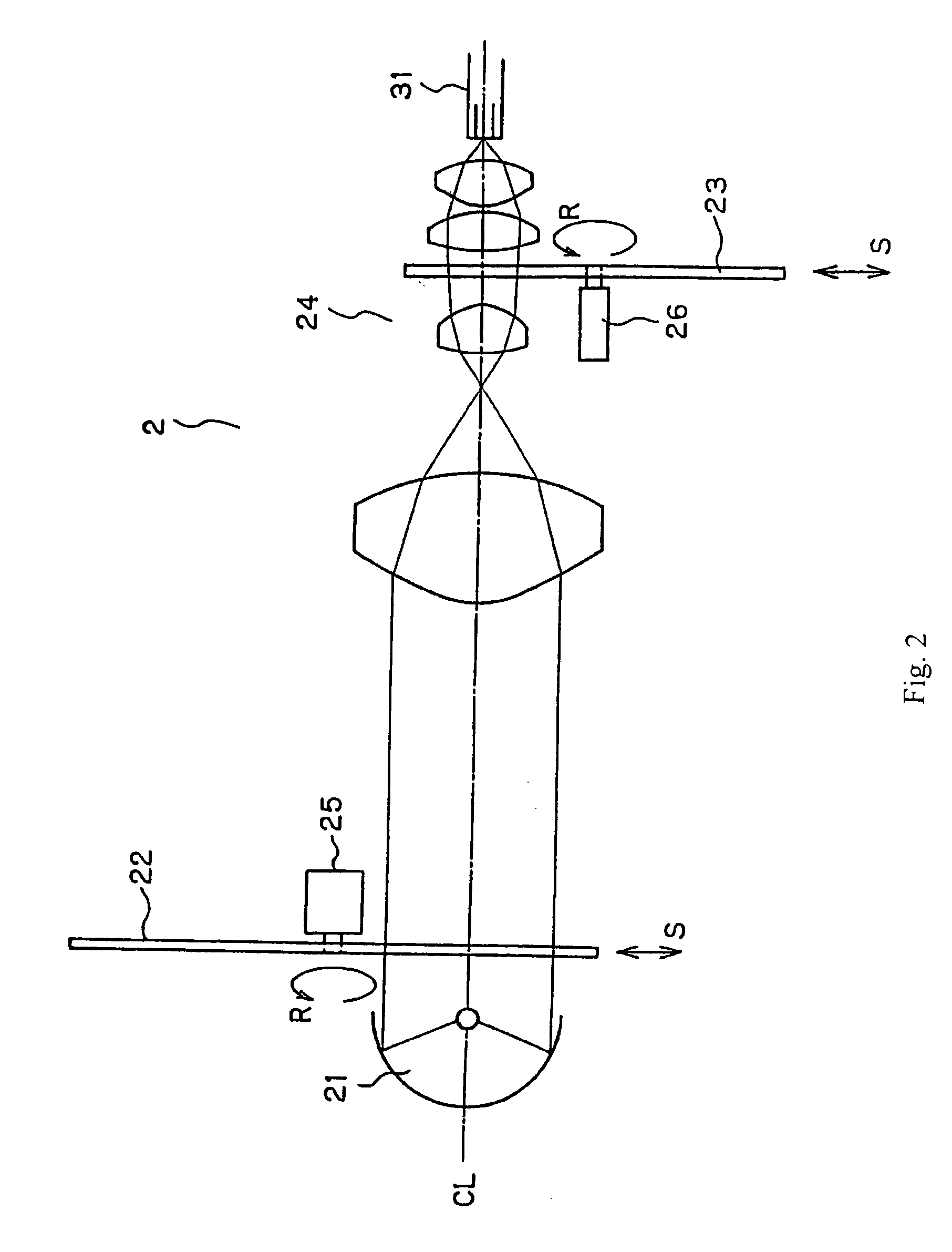
FIG. 2 is an illustration that shows the structure of the light source optical system in the light source system 2 which can be used to detect fluorescent labels such as quantum dots (having, for example, emission spectra as shown in FIG. 38) that have previously been introduced into living tissue 4 to be examined with the endoscope system of the present invention. The light source optical system 2 is formed of a light source 21, a turret 22 provided with plural optical filters, and a rotational disk 23 provided with plural optical filters that are arranged concentrically. The light source 21 can be a Xenon lamp that includes light wavele...
second embodiment
FIGS. 40-42 show alternative embodiments of the entire structure of the endoscope system according to the present invention wherein the structure that separates and detects plural fluorescent wavelengths is positioned other than in the endoscope tip. As the individual components are numbered identically with those of FIG. 1, only the differences will be now be described. FIG. 40 shows the overall structure of an endoscope system according to the present invention, characterized by having the components that separate and detect plural fluorescent wavelengths within the endoscope tip of the type that uses an optical fiber (fiberscope). Whereas in FIG. 1 the optical elements including the excitation light cut-off filter 34 are positioned just after the objective lens 33, in FIG. 40 a fiber bundle (a so-called image guide fiber bundle) is arranged just after the objective lens, and an ocular lens is provided at the exit side of the fiber bundle. The detection optical elements, which hav...
embodiment 1
FIG. 43 shows another embodiment of the present invention. Only the differences with regard to FIG. 43 will be described as compared to FIG. 39. In this embodiment, a tunable filter 35 and a detector 36 are used as in FIG. 1 as a wavelength separation element for separating fluorescent wavelengths emitted by the fluorescent labels in lieu of using a dichroic prism 125 and the three CCDs 124a, 124b, and 124c shown in FIG. 39. Furthermore, a color CCD 202 is used in lieu of the second dichroic prism 129 for detecting visible light components and the plural-circuit-board camera that uses the three CCDs 126, 127, and 128. Thus, the number of CCDs used at the camera head can be significantly reduced and this not only makes for a more compact design but also the endoscope has reduced cost. Also with this structure, the same detection ability of visible and fluorescent light as the endoscope system having the structure shown in FIG. 39 can be obtained. The color CCD 202 can be replaced by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com