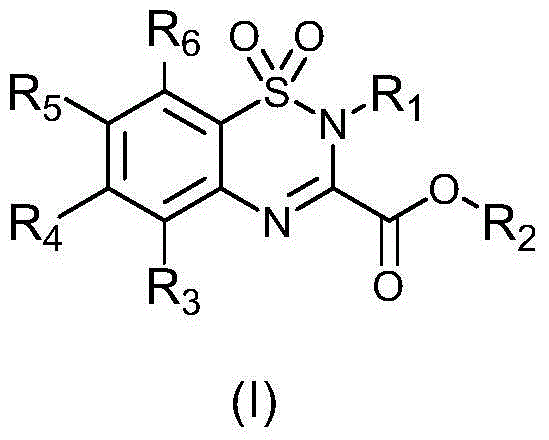
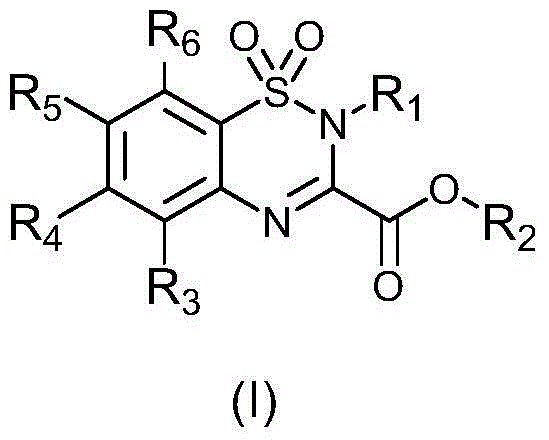
Carboxyl group-containing benzothiadiazide derivatives, as well as preparation method and application thereof
An alkyl and hydroxyl technology, applied in the field of benzothiadiazine derivatives, can solve problems such as inability to meet mass production, and achieve the effects of high yield, simple synthesis process and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
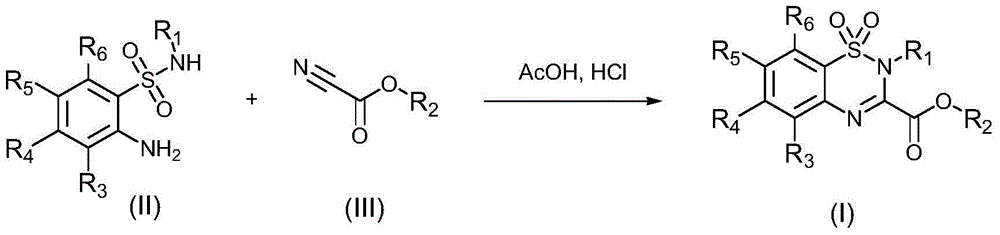
[0030] In the preparation method of any of the above-mentioned compounds, the synthetic route is as follows:
[0031]
[0032] The synthesis steps include: using compound (II) and compound (III) as raw materials, in the presence of acetic acid and concentrated hydrochloric acid, heating and reacting to obtain compound (I).
[0033] Wherein, the molar ratio of the compound (II) to the compound (III) is 1:(1-2), for example 1:1.5; the mass ratio of the acetic acid to concentrated hydrochloric acid is (5-15):1, more preferably (8-12):1, such as 10:1; the reaction temperature of the above heating reaction is 80-120° C., and the reaction time is at least 2 hours.
Embodiment 1
[0036] This embodiment provides a carboxyl-containing benzothiadiazine derivative compound (1):
[0037]
[0038] The synthetic route of this compound (1) is as follows:
[0039]
[0040] Synthetic steps include:
[0041] Dissolve sulfanilamide (20g, 0.19mol) and ethyl cyanoformate (26.3g, 0.29mol) in 100ml of acetic acid, gradually add 10ml of concentrated hydrochloric acid at room temperature, stir evenly, and heat to a temperature of 100°C. The reaction was carried out at this temperature for 5 hours. Take a small amount, add water and ethyl acetate, monitor the reaction result with TLC, the raw material aminobenzenesulfonamide has reacted completely. The reaction was stopped, most of the acetic acid was distilled off under reduced pressure, the remaining reaction liquid was cooled to room temperature, and water was added. Solids were precipitated, stirred evenly, filtered, and washed with water for 3 times. Vacuum drying gave a white solid product (19.1 g, yield:...
Embodiment 2
[0047] This embodiment provides a carboxyl-containing benzothiadiazine derivative compound (2):
[0048]
[0049] The synthetic route of this compound (2) is as follows:
[0050]
[0051] Synthetic steps include:
[0052] Dissolve sulfanilamide (20g, 0.19mol) and methyl cyanoformate (32.3g, 0.38mol) in 100ml of acetic acid, gradually add 10ml of concentrated hydrochloric acid at room temperature, stir evenly, and heat to a temperature of 100°C. The reaction was carried out at this temperature for 5 hours. Take a small amount, add water and ethyl acetate, monitor the reaction result with TLC, the raw material aminobenzenesulfonamide has reacted completely. The reaction was stopped, most of the acetic acid was distilled off under reduced pressure, the remaining reaction liquid was cooled to room temperature, and water was added. Solids were precipitated, stirred evenly, filtered, and washed with water for 3 times. Vacuum drying gave a white solid product (18.5 g, yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com