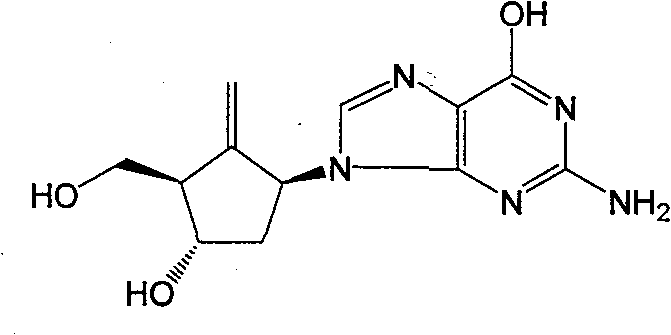
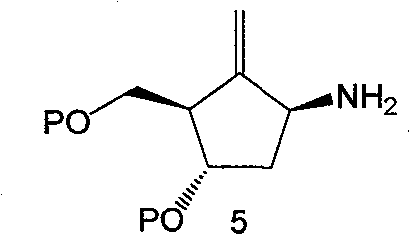
Synthesis method of antiviral nucleoside analogue
A compound, the technology of entecavir, which is applied in the field of intermediates for the preparation of entecavir, can solve the problems of high cost, unsuitability, difficult separation, complicated process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: [1s-(1α, 2β, 3α, 5β)]-5-(phthalimide)-3-(benzyloxy)-2-[(benzyloxy)methyl base] cyclopentanol (intermediate 2') preparation
[0065] In a 5L three-neck flask, add 145g (0.98mol) of phthalimide, 3.77g of LiH and 765ml of anhydrous DMF, and stir for 10min. After heating to 60°C, stir for 15 minutes, at which time the cloudy solution becomes clear. Slowly drop 152g (0.49mol) [1s-(1α, 2α, 3β, 5α)]-3-(benzyloxy)-2-[(benzyloxy)methyl] dissolved in 1.87L of anhydrous DMF -6-Oxabicyclo[3.1.0]hexane (intermediate 1'), stirred at 60°C for 15min. Heat to 125°C, react for 2 hours, TLC (B:positive=1:3) shows that the raw material disappears, and terminate the reaction with 28ml of glacial acetic acid. Stir for 10 min. Add 2.5 L of saturated brine, extract with ethyl acetate 3×1.2 L, combine the organic phases, wash once with saturated brine, dry over anhydrous sodium sulfate, and recover the solvent. The remaining oil was separated on a silica gel column and eluted wi...
Embodiment 2
[0069] Example 2: [1s-(1α, 2β, 3α, 5β)]-5-[phthalimide]-3-(benzyloxy)-2-[(benzyloxy)methyl Base] the preparation of cyclopentanone (intermediate 3')
[0070] In a 3L three-neck flask, add 203g of Dess-Martin reagent, add 1.4L of anhydrous CH 2 Cl 2 Stir. 137.7g intermediate 2' was dissolved in 890ml anhydrous CH 2 Cl 2 Dissolve and add dropwise to the suspension in the previous step. After 20 min, TLC (B: positive = 1:3) showed that the raw material disappeared, and the reaction was stopped. NaHSO 3 Washed three times with saturated aqueous solution, and then washed with NaHCO 3 Washed with saturated aqueous solution for 3 times, and finally washed with saturated brine for 3 times, and the organic layer was dehydrated and dried to obtain 196 g of a yellow oily compound.
Embodiment 3
[0071] Example 3: 1s-(1α, 3α, 4β)-5-phthalimido-2-methylene-4-(benzyloxy)-3-[(benzyloxy)methanol Preparation of Cyclopentane (Intermediate 4')
[0072] In a 5L three-necked flask, add Nysted Reagent (Wt=20%) 1.46L and 800ml anhydrous THF, stir, N 2 Protected and cooled to -78°C. 196g intermediate 3' with appropriate amount of CH 2 Cl 2 Dissolved and added dropwise to the reaction. Take TiCl 4 / CH 2 Cl 2 (1:9) 393ml was slowly added dropwise to the reaction, maintaining the temperature at -60°C to -78°C. After the dropwise addition was completed, the mixture was maintained at -78°C for 15 min. Slowly warm up to room temperature, and continue to stir for 1-3 hours. TLC (B: positive = 1:4) showed that the raw materials disappeared, and the reaction solution was purple-black. Pour this reaction solution into 2.3L saturated NaHCO 3 In the middle, stir thoroughly, and white turbidity will appear at this time. Extracted three times with ethyl acetate, back-extracted once w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com