Injection type directional sustained-release paeonol thermosensitive gel and its preparation method
A phenol temperature-sensitive gel and injection-type technology, which is applied in the field of injection-type sustained-release paeonol temperature-sensitive gel and its preparation, can solve problems such as the inability to guarantee long-term effective drug concentration at the patient site, and achieve long-lasting drug effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
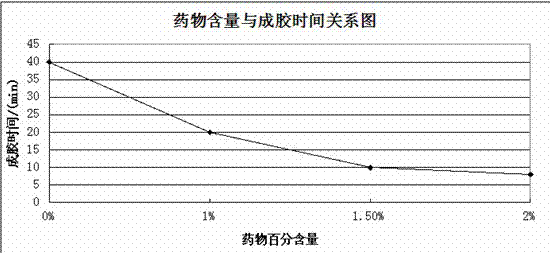
[0025] An injection-type directional sustained-release paeonol temperature-sensitive gel is formed by dissolving paeonol in a water-based solution and solidifying it. The water-based solution is composed of chitosan, sodium β-glycerophosphate, hydrochloric acid solution, and sodium hydroxide solution. Prepared with deionized water, wherein the ratio of chitosan, β-sodium glycerophosphate, hydrochloric acid solution and deionized water is 85mg: 0.45ml: 450mg: 0.45ml;
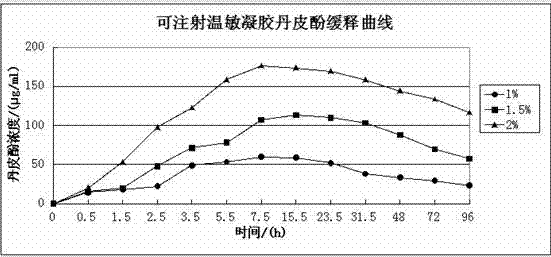
[0026] The ratio of paeonol to the water-based solution is that 1 g of paeonol is added to every 99 g of the water-based solution, that is, the mass percentage of paeonol in the cured composite hydrogel is 1%.
[0027] The preparation method of the above-mentioned injection-type directional slow-release paeonol thermosensitive gel comprises the following steps:
[0028] 1) Weigh chitosan, sodium β-glycerophosphate, hydrochloric acid solution, sodium hydroxide solution and deionized water according to the above ra...
Embodiment 2
[0047] An injection-type directional sustained-release paeonol temperature-sensitive gel is formed by dissolving paeonol in a water-based solution and solidifying it. The water-based solution is composed of chitosan, sodium β-glycerophosphate, hydrochloric acid solution, and sodium hydroxide solution. Prepared with deionized water, wherein the ratio of chitosan, β-sodium glycerophosphate, hydrochloric acid solution and deionized water is 90mg: 0.5ml: 500mg: 0.5ml;
[0048] The ratio of paeonol to the water-based solution is that 1.5 g of paeonol is added to every 98.5 g of the water-based solution, that is, the mass percentage of paeonol in the cured composite hydrogel is 1.5%.
[0049] The preparation method of the above-mentioned injection-type directional slow-release paeonol thermosensitive gel comprises the following steps:
[0050] 1) Weigh chitosan, sodium β-glycerophosphate, hydrochloric acid solution, sodium hydroxide solution and deionized water according to the abov...
Embodiment 3
[0058] An injection-type directional sustained-release paeonol temperature-sensitive gel is formed by dissolving paeonol in a water-based solution and solidifying it. The water-based solution is composed of chitosan, sodium β-glycerophosphate, hydrochloric acid solution, and sodium hydroxide solution. Prepared with deionized water, wherein the ratio of chitosan, β-sodium glycerophosphate, hydrochloric acid solution and deionized water is 95mg: 0.55ml: 550mg: 0.55ml;
[0059] The ratio of paeonol to the water-based solution is that 2g of paeonol is added to every 98g of the water-based solution, that is, the mass percentage of paeonol in the cured composite hydrogel is 2%.
[0060] The preparation method of the above-mentioned injection-type directional slow-release paeonol thermosensitive gel comprises the following steps:
[0061] 1) Weigh chitosan, sodium β-glycerophosphate, hydrochloric acid solution, sodium hydroxide solution and deionized water according to the above rati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com