Methotrexate chitosan thermosensitive gel and its application
A technology of methotrexate shell and temperature-sensitive gel is applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, organic active ingredients, etc. The effect of quality, controllable quality and stable nature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The construction of embodiment 1 methotrexate-chitosan thermosensitive gel
[0051] 1 Method and results
[0052] 1.1 Solution preparation
[0053] Raw materials and dosage:
[0054]
[0055] 1.1.1 Chitosan solution
[0056] Weigh the prescribed amount of CS, add an appropriate amount of water, stir magnetically to form a CS suspension, then add the prescribed amount of acidic solvent drop by drop, and continue stirring to completely dissolve the CS to obtain a CS solution.
[0057] 1.1.2 Disodium glycerophosphate solution
[0058] Weigh the prescribed amount of GP, add appropriate amount of water, and ultrasonically dissolve to obtain the GP solution.
[0059] 1.1.3 Methotrexate solution
[0060] Weigh the prescribed amount of MTX and other excipients (NaOH, solubilizer, isotonicity regulator), add appropriate amount of water, and ultrasonically dissolve to obtain the MTX solution.
[0061] The above-prepared solution was stored at 4°C for future use.
[0062...
Embodiment 2
[0133] Example 2 Methotrexate Chitosan Thermosensitive Gel Formulation Properties Research
[0134] 1 Method and results
[0135] 1.1 Appearance
[0136] CS / GP / MTXsol is a light yellow transparent solution, no insoluble components or massive agglomerates can be seen by naked eyes. CS / GP / MTXsol was gelled in a 37°C water bath to obtain CS / GP / MTXgel, which was in the form of light yellow semi-solid.
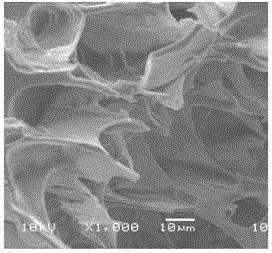
[0137] 1.2 Microstructure of the gel
[0138] After the CS / GP / MTXgel sample was freeze-dried for 24 hours, it was snapped off by liquid nitrogen quenching, and its cross-section was vacuum-sprayed with gold. The internal structure of CS / GP / MTXgel was characterized by SEM. It can be seen from Figure 1 that the internal structure of CS / GP / MTXgel is irregular and porous.
[0139] 1.3 Viscosity, gel time and pH value
[0140] Prepare 3 batches of CS / GP / MTXsol according to the optimized prescription, and measure their t gel , η and pH value, see Table 2-1. The results show that t...
Embodiment 3
[0190] Example 3 Pharmacokinetics of Methotrexate Chitosan Thermosensitive Gel in Rabbits
[0191] 1.1 Experimental animals
[0192] Healthy male New Zealand white rabbits (1.8-2.5Kg), license number: SCXK (Xiang) 2009-0012, Changsha Tianqin Biotechnology Co., Ltd.
[0193] 2 Methods and results
[0194] 2.1 Reagent
[0195] 2.1.1 Preparation of CS / GP / MTXsol
[0196] With embodiment 1.
[0197] 2.1.2 Preparation of methotrexate injection
[0198] Weigh an appropriate amount of NaOH, and prepare a concentration of 0.198mol L with 0.9% NaCl solution -1 NaOH saline solution. Use NaOH physiological saline solution to prepare a concentration of 50mg·mL -1 MTX injection.
[0199] 2.2 Experimental scheme
[0200] Twelve New Zealand white rabbits were randomly divided into two groups (n=6), namely the experimental group and the control group. According to 5mg·kg -1 Subcutaneous injection on the back, the experimental group was injected with CS / GP / MTXsol, and the control gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com