Lithium carbonate sustained release tablet
A technology of sustained-release tablets and lithium carbonate, which is applied in the directions of inactive medical preparations, pill delivery, inorganic active ingredients, etc., can solve problems such as unfavorable industrialized large-scale production, excessive drying time, and excessive dust, and achieve long-lasting effects. , The effect of stable curative effect and reduction of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
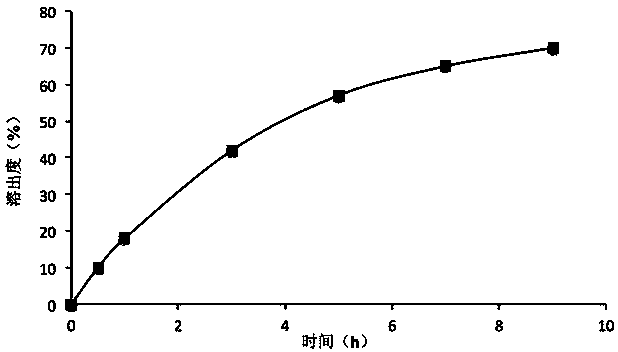
Embodiment 1
[0024] prescription:
[0025]
[0026] Preparation method: pass lithium carbonate through a 80-mesh sieve, and sieve hypromellose, lactose, stearic acid, magnesium stearate, and talcum powder through a 60-mesh sieve; take the prescription amount of lithium carbonate, hypromellose, lactose, hard Fatty acids are mixed evenly, granulated with 7.5% polyvinylpyrrolidone 75% ethanol solution containing Tween 80, dried in a fluidized bed, passed through a 20-mesh sieve for granulation, added magnesium stearate and talcum powder, mixed and compressed into tablets.
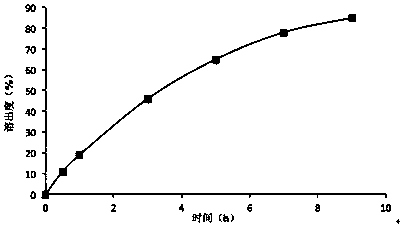
Embodiment 2
[0028] prescription:
[0029]
[0030] Preparation method: pass lithium carbonate through 80 mesh sieve, hypromellose K4M, hypromellose K15M, microcrystalline cellulose, stearic acid, sodium lauryl sulfate, calcium stearate, micropowder silica gel through 60 Mesh sieve; take prescription amount of lithium carbonate, hypromellose K4M, hypromellose K15M, microcrystalline cellulose, sodium lauryl sulfate, stearic acid and mix evenly, granulate with 75% ethanol solution, boil Bed-dried, passed through a 20-mesh sieve for granulation, added calcium stearate and micropowder silica gel, mixed and pressed into tablets.
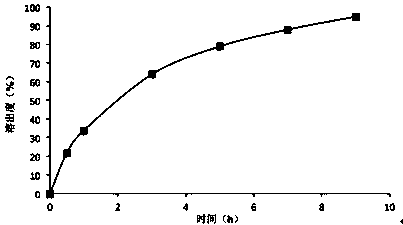
Embodiment 3
[0032] prescription:
[0033]
[0034] Preparation method: Pass lithium carbonate through a 80-mesh sieve, hydroxyethyl cellulose, calcium sulfate, magnesium stearate, and micropowder silica gel pass through a 60-mesh sieve; take the prescription amount of lithium carbonate, hydroxyethyl cellulose, and calcium sulfate and mix evenly, The lecithin-containing 5% polyvinylpyrrolidone 75% ethanol solution is granulated, dried in a fluidized bed, sieved through a 20-mesh sieve for granulation, added magnesium stearate and micropowder silica gel to mix and then compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com