Method of preparing polymer electrospinning fiber and application in transdermal drug delivery patch
An electrospinning fiber and polymer technology, applied in the application of transdermal drug patch, the field of polymer electrospinning fiber preparation, can solve the problems of improving, not using, not involving the compatibility of pressure-sensitive adhesives, etc., to promote the Effects of drug release, improved stability, improved performance and scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
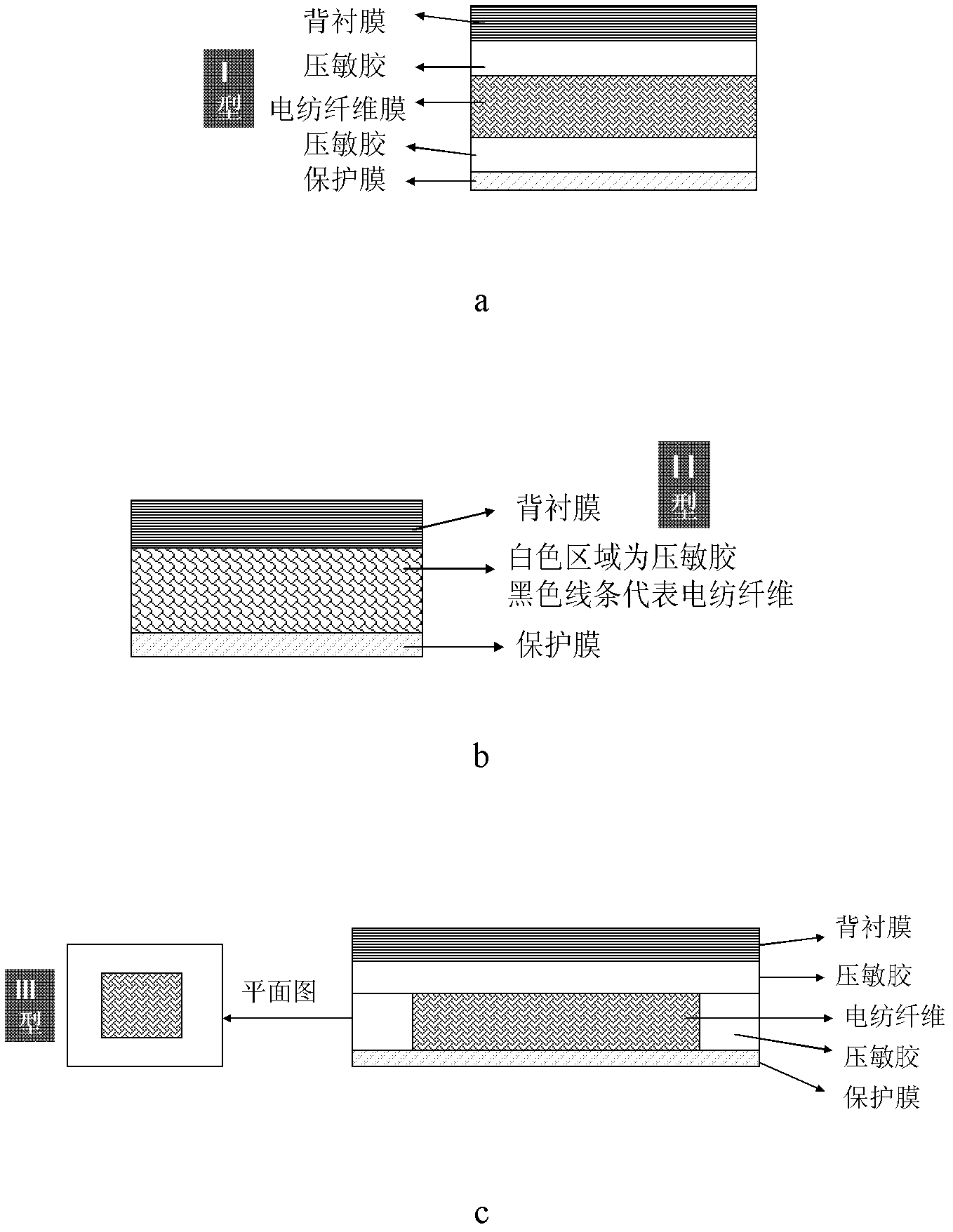
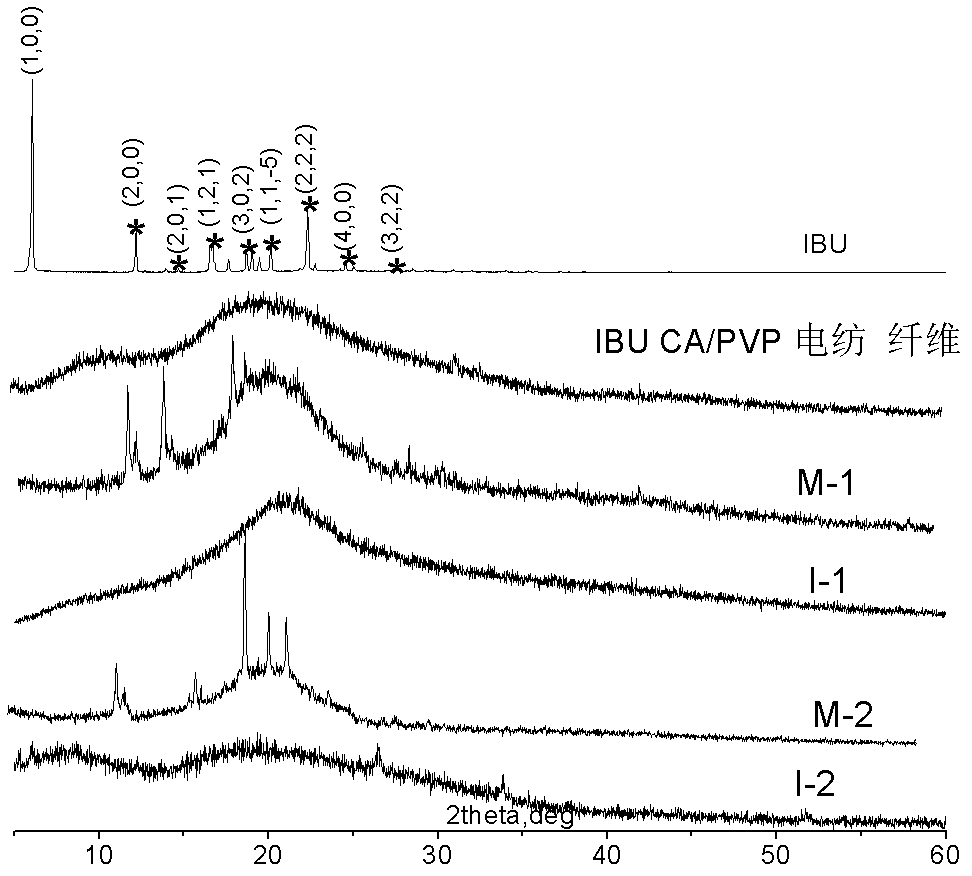
[0033] Preparation of polymer electrospinning fiber solution: N,N-dimethylacetamide / acetone (V:V =2:1, volume ratio) solution, then add 20% ibuprofen and 20% azone (accounting for the mass fraction of the electrospun polymer), dissolve evenly, and then spin. The electrospinning conditions were as follows: needle No. 8, voltage 13KV, spray speed 0.5ml / h, curing distance 20cm; aluminum foil was used to receive and spin the drug-loaded electrospun fiber membrane, and the prepared electrospun fiber membrane was dried in vacuum for 24 hours. The prepared electrospun fiber membrane was vacuum dried for 24 h. A non-woven fabric coated with polyacrylate pressure-sensitive adhesive (thickness 0.1-1mm) is used as a backing film, and then a quantitative amount of the drug-loaded electrospun fiber membrane prepared above is attached to the backing film, and then coated with 2.5 The release paper protective film of the PSA2 pressure-sensitive adhesive layer (thickness 0.1-2mm) of % ibupro...
Embodiment 2
[0038] According to the method of Example 1, only the pressure-sensitive adhesive layer is changed to PSA1 pressure-sensitive adhesive layer to obtain patch I-2.
[0039] The PSA1 pressure-sensitive adhesive containing 5% ibuprofen and 5% azone was directly coated on the release paper to form the pressure-sensitive adhesive monolithic patch M-2 as a control.
[0040] Ibuprofen and azone are dissolved into the N,N-dimethylacetamide / acetone (V: V=2:1, volume ratio) solution, cast to form a film, use it to replace the electrospun film in I-2, and combine it with PSA1 pressure-sensitive adhesive to form patch F-2, as a control.
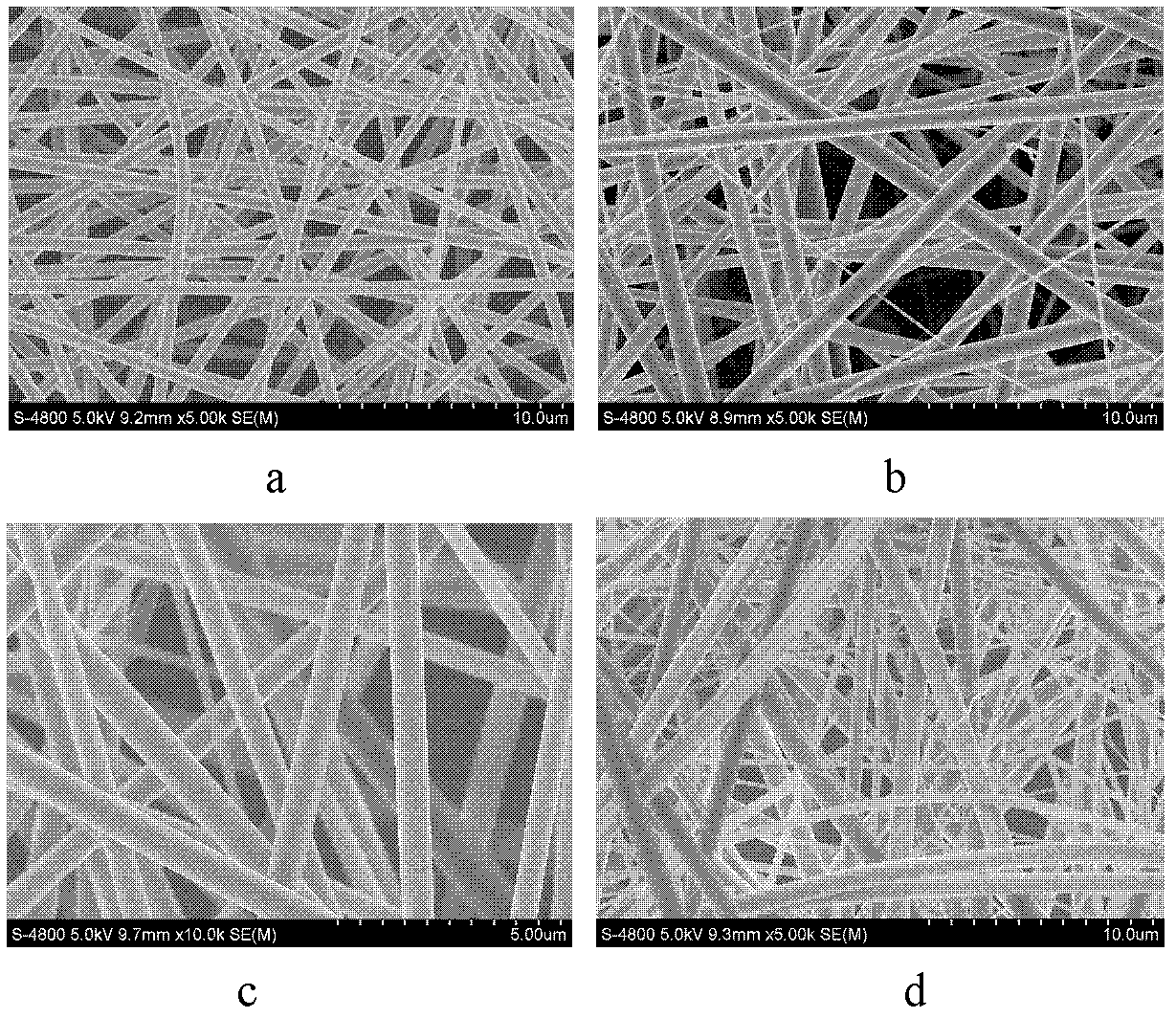
[0041] For the characterization of the dispersion state of the drug in patch I-2, see image 3 and 4 , no drug crystallization; see Tables 4, 5 and 6 for the adhesiveness, storage stability and transdermal penetration rate of patches I-2, F-2 and M-2.
Embodiment 3
[0043] Prepare an ethanol solution of 15% polyvinylpyrrolidone (PVPK90) containing 20% methyl nicotinate (accounting for the mass fraction of PVP, the same below), and carry out spinning. The electrospinning conditions are: needle No. 8, voltage 10KV, The spray speed was 0.5ml / h, and the curing distance was 20cm; aluminum foil was used to receive and spin to form drug-loaded electrospun fiber membranes, and the prepared electrospun fiber membranes were vacuum-dried for 24 hours. The non-woven fabric is used as the backing film, and a layer of polyacrylate pressure-sensitive adhesive (thickness 0.1-1mm) is coated on the top, the drug-loaded electrospun fiber membrane prepared above is attached to the pressure-sensitive adhesive, and then coated with PSA2 A release paper protective film with a pressure-sensitive adhesive layer (thickness 0.1-2mm), rolled to form an I-type patch I-3. The adhesiveness of the patch is shown in Table 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com