Application of ionic type cyclodextrin derivative in preparation of medicine preparation for iontophoresis transdermal administration
A pharmaceutical preparation and iontophoresis technology, which is applied in drug delivery, non-active ingredients of polymer compounds, pharmaceutical formulations, etc., can solve the problems of limited transdermal penetration of drugs, and achieve increased transdermal penetration rate, enhanced electric field response, The effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
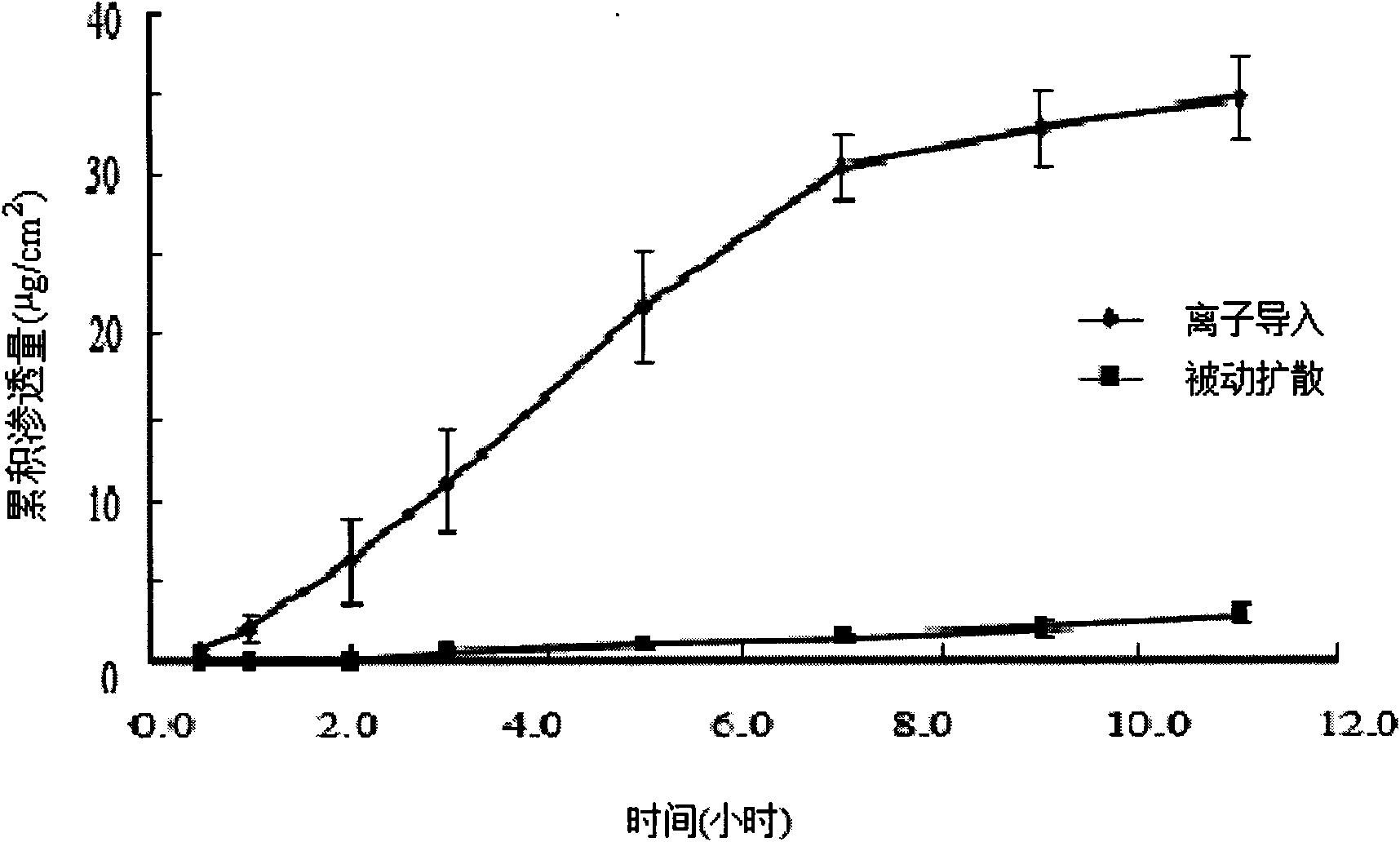
[0030] Promotion of Hydrocortisone (HS) Iontophoresis by 5% Sulfobutyl-β-cyclodextrin (SBE-CD)
[0031] 1 g of excess hydrocortisone was placed in 100 ml of SBE-CD phosphate buffered solution (PBS) with a mass percent concentration of SBE-CD of 5%, and magnetically stirred to form a HS-SBE-CD clathrate solution.
[0032] Another 1 g of hydrocortisone was placed in 100 ml of phosphate buffered solution (PBS), and magnetically stirred evenly to form a control solution.
[0033] Take the skin of the rat body from which the subcutaneous tissue has been removed, and fix it between the supply pool and the receiving pool of the horizontal transdermal two-chamber iontophoresis diffusion cell, exhaust the air bubbles, and keep the circulating water at (32±0.5)°C. Place the above-mentioned HS-SBE-CD clathrate solution or control solution in the supply tank, use PBS as the receiving solution in the receiving tank, add a stirrer to stir at a speed of 500r / min, the electrodes are all Pt, the...
Embodiment 2
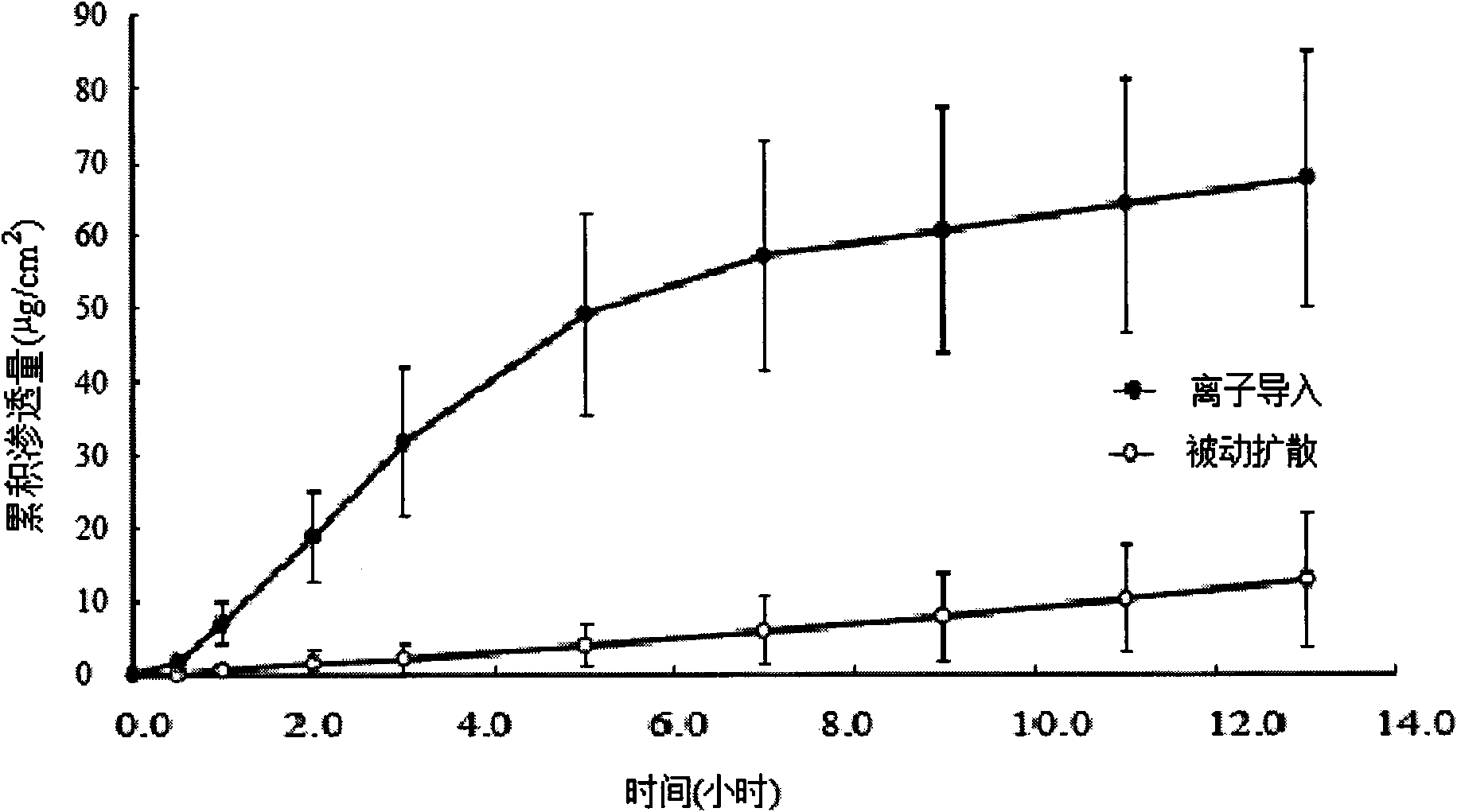
[0039] Promotion of Hydrocortisone (HS) Iontophoresis by 10% Sulfobutyl-β-cyclodextrin (SBE-CD)
[0040] 2 g of excess hydrocortisone was placed in 100 ml of SBE-CD phosphate buffer solution (PBS) with a concentration of 10% by mass of SBE-CD, and magnetically stirred to form a HS-SBE-CD clathrate solution.
[0041] Another 2 g of hydrocortisone was placed in 100 ml of phosphate buffered solution (PBS), and magnetically stirred evenly to form a control solution.
[0042] Take the skin of the rat body from which the subcutaneous tissue has been removed, and fix it between the supply pool and the receiving pool of the horizontal transdermal two-chamber iontophoresis diffusion cell, exhaust the air bubbles, and keep the circulating water at (32±0.5)°C. Place the above-mentioned HS-SBE-CD clathrate solution or control solution in the supply tank, use PBS as the receiving solution in the receiving tank, add a stirrer to stir at a speed of 500r / min, the electrodes are all Pt, the ne...
Embodiment 3
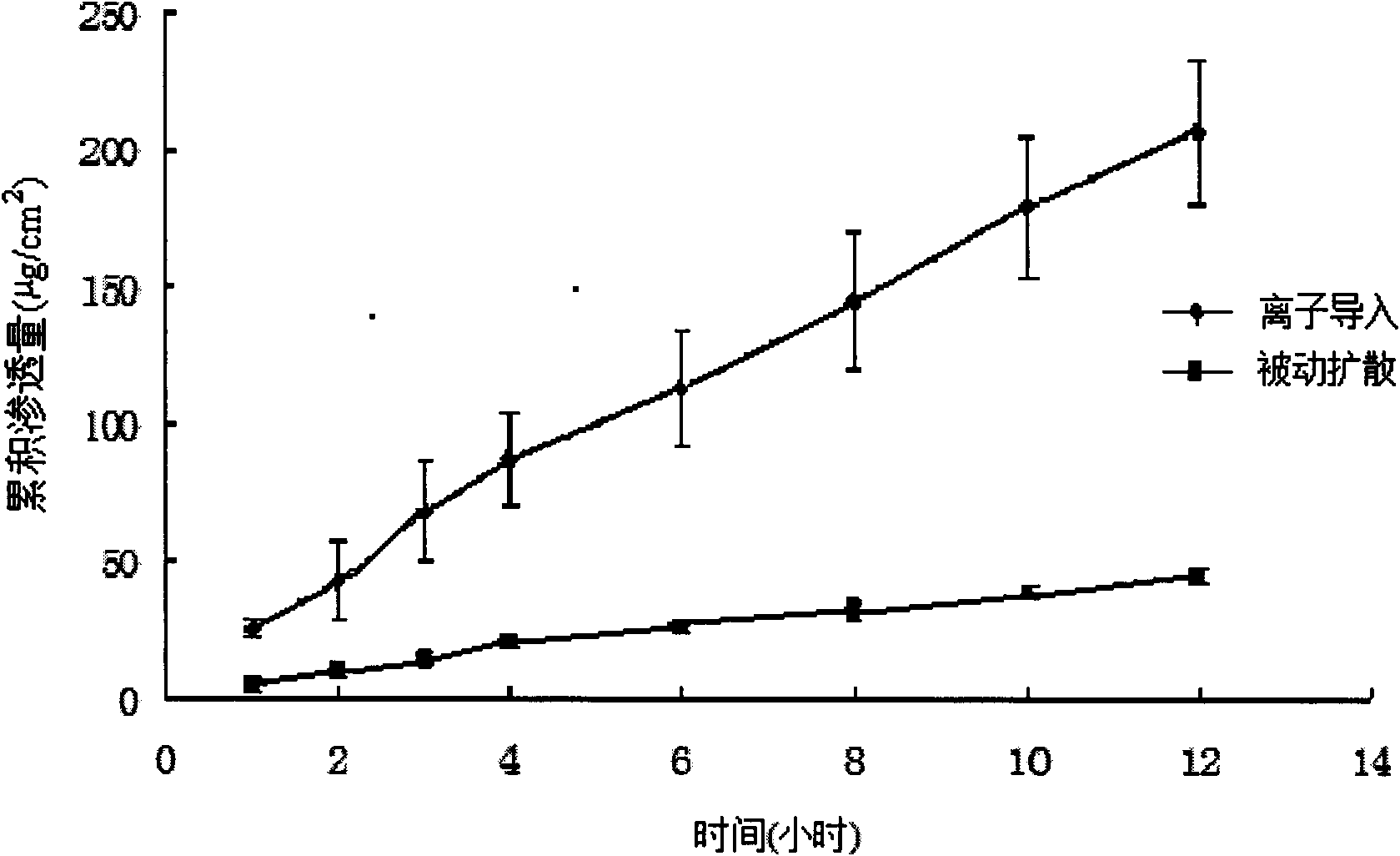
[0048] The promotion of iontophoresis of estradiol (ES) by sulfobutyl-β-cyclodextrin (SBE-CD)
[0049] 1.5 g of excess estradiol was placed in 10 ml of SBE-CD phosphate buffer solution (PBS) with a concentration of 5% by mass of SBE-CD, and magnetically stirred to form an ES-SBE-CD clathrate solution.
[0050] Take the skin of the rat body from which the subcutaneous tissue has been removed, and fix it between the supply pool and the receiving pool of the horizontal transdermal two-chamber iontophoresis diffusion cell, exhaust the air bubbles, and keep the circulating water at (32±0.5)°C. Place the above-mentioned ES-SBE-CD clathrate solution in the supply tank, use PBS as the receiving solution in the receiving tank, add a stirrer to stir at a speed of 500r / min, the electrodes are all Pt, the negative electrode is introduced, and the current intensity is 0.4mA / 0.79 cm 2 , for iontophoresis. Take 1ml of receiving solution at 1h, 2h, 3h, 4h, 6h, 8h, 10h and 12h respectively, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com