Preparation method and application of galvanic-response transdermal drug delivery system
A transdermal drug delivery system and a technology for transdermal drug delivery are applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients to improve the transdermal penetration rate, physical and chemical stability. The effect of good, easy-to-satisfy preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation and Characterization of Carboxylated Carbon Nanotubes
[0044] Commercially purchased MWCNTs (500 mg) were dispersed in 500 mL 98% H 2 SO 4 and 65% HNO 3 Add the mixed acid (3:1, v / v) into a ball mill tank, protect it with liquid nitrogen, and ball mill for 20 minutes at a speed of 590 rpm. Wash with ultrapure water (18.2MΩ) to neutrality, collect after suction filtration (0.45μm vinylidene chloride microporous filter membrane), and vacuum dry at 60°C for 24h to obtain carboxylated carbon nanotubes (MWCNTs-COOH).
[0045] In a 250 mL single-neck round bottom flask equipped with a magnetic stirring rotor, add the above-mentioned oxidized MWCNTs-COOH (40 mg) and dilute nitric acid (2.6 mol L -1 , 120mL), after ultrasonic treatment for 30min, put it into an oil bath and install a spherical condenser and tail gas absorption tube, heat and reflux and stir for 24h. After the reaction, cool, wash with ultrapure water until neutral and collect, and va...
Embodiment 2
[0049] Example 2: Preparation of Aceclofenac (Aceclofenac, abbreviated as AC) electroreactive transdermal drug delivery system
[0050] Accurately weigh AC to make 25mg·mL -1 (95% ethanol) solution 30mL, set aside. Weigh 500mg of MWCNTs-COOH and place it in a ball mill jar, add the liquid medicine in 3 times, 10mL each time; 590rpm·min under the protection of liquid nitrogen -1 Ball mill for 6 minutes. The suspension was taken out and passed through a 0.45 μm microporous membrane, the filter cake was washed and filtered with deionized water, and then freeze-dried to obtain the carboxylated multi-walled carbon nanotube-loaded aceclofenac complex (AC-MWCNTs-COOH) .
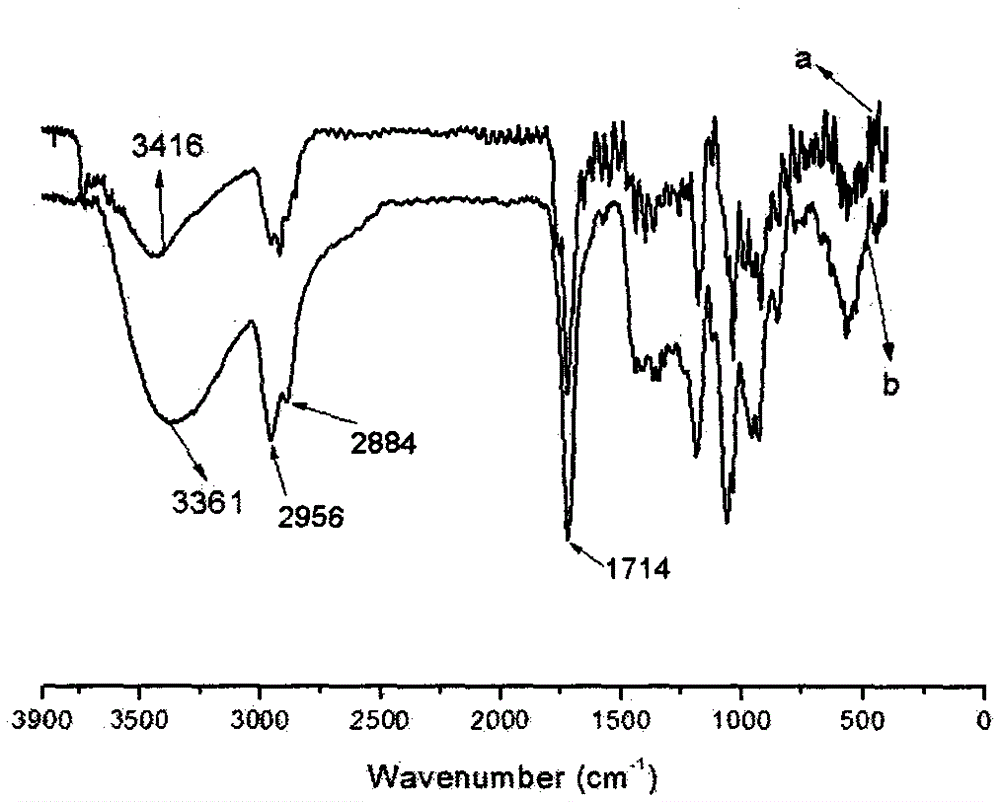
[0051] Infrared characterization of AC-MWCNTs-COOH, see description Figure 4 As shown (A, B, and C in the figure are the infrared spectra of the aceclofenac raw material drug, the load AC-MWCNTs-COOH, and the carboxylated carbon nanotube MWCNTs-COOH respectively), the carboxyl peak of the aceclofenac raw materi...
Embodiment 3
[0053] Embodiment 3: Determination of the transdermal rate of aceclofenac electroreactive transdermal drug delivery system
[0054] In order to compare the transdermal rate of aceclofenac electroreactive transdermal drug delivery system (AC-MWCNTs-COOH gel ointment) and common aceclofenac gel ointment (AC gel ointment), the AC gel ointment was first prepared, The difference between the preparation method and process lies in that AC is the raw material drug, and is not loaded with carboxylated carbon nanotubes; no carbon nanotubes are added to the gel matrix.
[0055] Specific operation steps: put 1g 10 was sprinkled in 15 mL of deionized water, and stirred at a low speed for 30 min; weighed 0.5 g of AC, added 5 mL of 95% ethanol to dissolve, and added to the carbomer solution under stirring to obtain a phase A solution. In phase B, weigh 5.5g of NP-700, add 20mL of glycerin, 4mL of propylene glycol, and 4mL of azone, and grind well. Dissolve 2 g of sorbitol and 0.01 g of ED...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com