Plant extract-mediated drug transdermal introducing system and transdermal method thereof
A plant extract and transdermal technology, applied in drug combination, drug delivery, pharmaceutical formulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
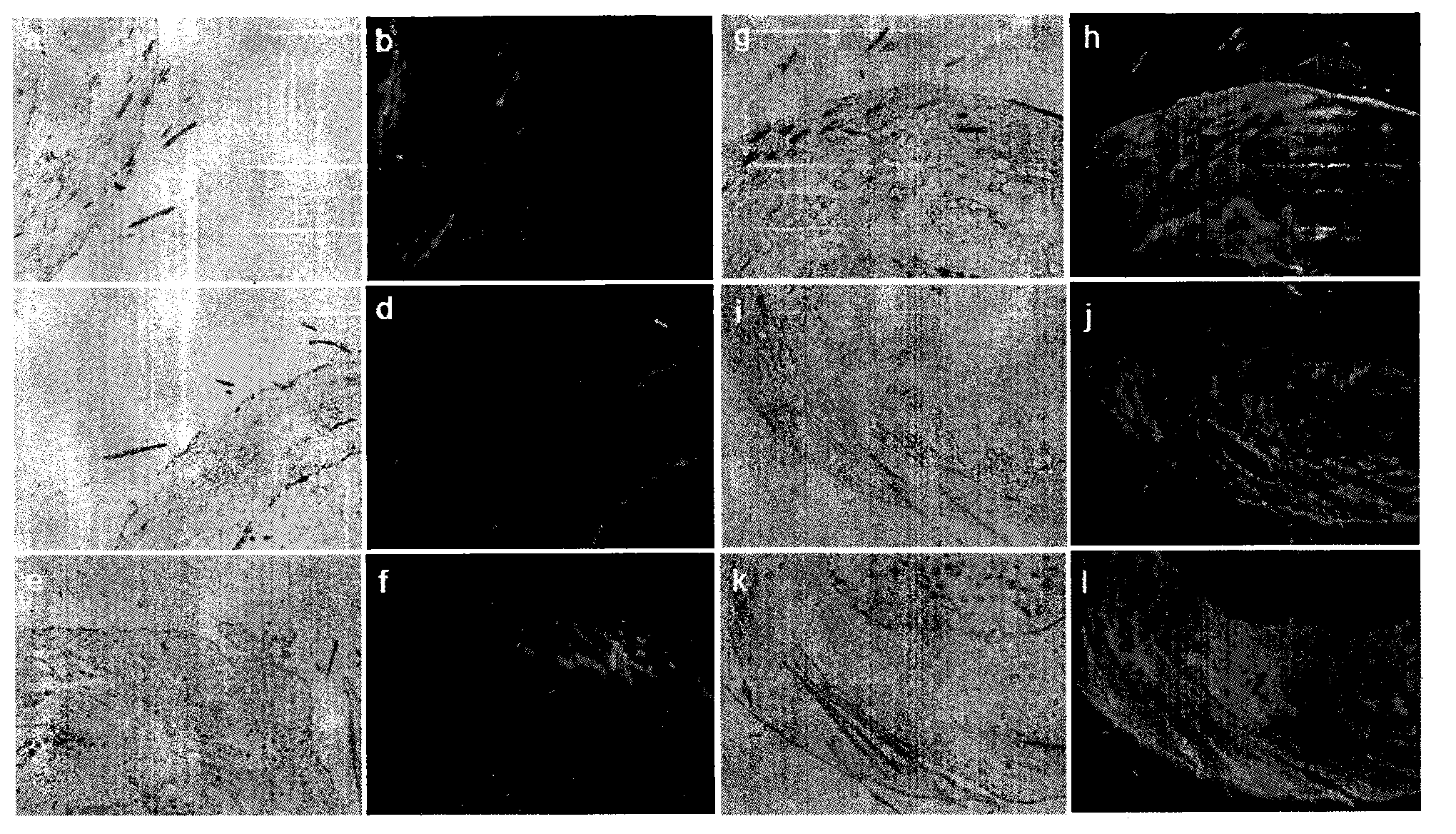
[0127] Animal skin penetration enhancement experiment
[0128] 1. Main instruments, reagents and materials
[0129] 1.1 ICR mice: Experimental Animal Center of Nantong University, 4-6 weeks old, weighing 18-20 g, male and female randomly.
[0130] 1.2 Fluorescence-labeled siRNA: Cy3-labeled siRNA (Cy3-siRNA) (Biomicro Biotechnology Co., Ltd.).
[0131] 1.3 Instruments: cryostat (Leica CM1900, Leica, USA); upright fluorescence microscope (X3, Olympus, Japan), etc.
[0132] 1.4 Reagent: 10% (m / V) chloral hydrate (Sinopharm Chemical Reagent Co., Ltd.); medical glue (Guangzhou Baiyun Medical Glue Co., Ltd.); sodium chloride injection (0.9%) (Shandong Kangning Pharmaceutical Co., Ltd.) ; 0.1MPBS (BIOMEC Biotechnology Co., Ltd.); Angelica Essential Oil (Jiangxi Cedar Natural Medicinal Oil Co., Ltd.); Azone (Aladdin Reagent (China) Co., Ltd.).
[0133]1.5 Materials: 1mL disposable syringe, 5mL disposable syringe (Jiangyan Xinsu Medical Instrument Co., Ltd.), disposable cotton swab...
Embodiment 2
[0149] Animal skin transdermal experiment
[0150] 1. Main instruments, reagents and materials
[0151] 1.1 ICR mice: Experimental Animal Center of Nantong University, 4-6 weeks old, weighing 18-20 g, male and female randomly.
[0152] 1.2 Fluorescence-labeled siRNA: Cy3-labeled siRNA (Cy3-siRNA) (Biomicro Biotechnology Co., Ltd.).
[0153] 1.3 Instruments: cryostat (Leica CM1900, Leica, USA); upright fluorescence microscope (X3, Olympus, Japan), etc.
[0154] 1.4 Reagent: 10% (m / V) chloral hydrate (Sinopharm Chemical Reagent Co., Ltd.); medical glue (Guangzhou Baiyun Medical Glue Co., Ltd.); sodium chloride injection (0.9%) (Shandong Kangning Pharmaceutical Co., Ltd.) ; 0.1MPBS (BIOMEC Biotechnology Co., Ltd.); Angelica Essential Oil (Jiangxi Cedar Natural Medicinal Oil Co., Ltd.); Azone (Aladdin Reagent (China) Co., Ltd.).
[0155] 1.5 Materials: 1mL disposable syringe, 5mL disposable syringe (Jiangyan Xinsu Medical Instrument Co., Ltd.), disposable cotton swab, 5mL centr...
Embodiment 3
[0168] Concentration Dependence Experiment of Nucleic Acid Molecule Transdermal Transdermal Drug
[0169] 1. Main instruments, reagents and materials
[0170] 1.1 ICR mice: Experimental Animal Center of Nantong University, 4-6 weeks old, weighing 18-20 g, male and female randomly.
[0171] 1.2 Fluorescence-labeled siRNA: Cy3-labeled siRNA (Cy3-siRNA) (Biomicro Biotechnology Co., Ltd.).
[0172] 1.3 Instruments: cryostat (Leica CM1900, Leica, USA); upright fluorescence microscope (X3, Olympus, Japan), etc.
[0173] 1.4 Reagent: 10% (m / V) chloral hydrate (Sinopharm Chemical Reagent Co., Ltd.); Sodium chloride injection (0.9%) (Shandong Kangning Pharmaceutical Co., Ltd.); Co., Ltd.); Angelica Essential Oil (Jiangxi Cedar Natural Medicinal Oil Co., Ltd.); Jojoba Oil (Zibo Linsen Biological Products Co., Ltd.), etc.
[0174] 1.5 Materials: 1mL disposable syringe, 5mL disposable syringe (Jiangyan Xinsu Medical Instrument Co., Ltd.), disposable cotton swab, 5mL centrifuge tube, op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com