Transdermal delivery rate control using amorphous pharmaceutical compositions
a technology of compositions and pharmaceuticals, applied in the direction of aerosol delivery, extracellular fluid disorder, metabolism disorder, etc., can solve the problems of complex mechanism of transdermal drug delivery, complicated oral administration route, and increased skin irritation, so as to reduce the percutaneous absorption efficiency and increase the propensity to skin irritation. , the effect of increasing the energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
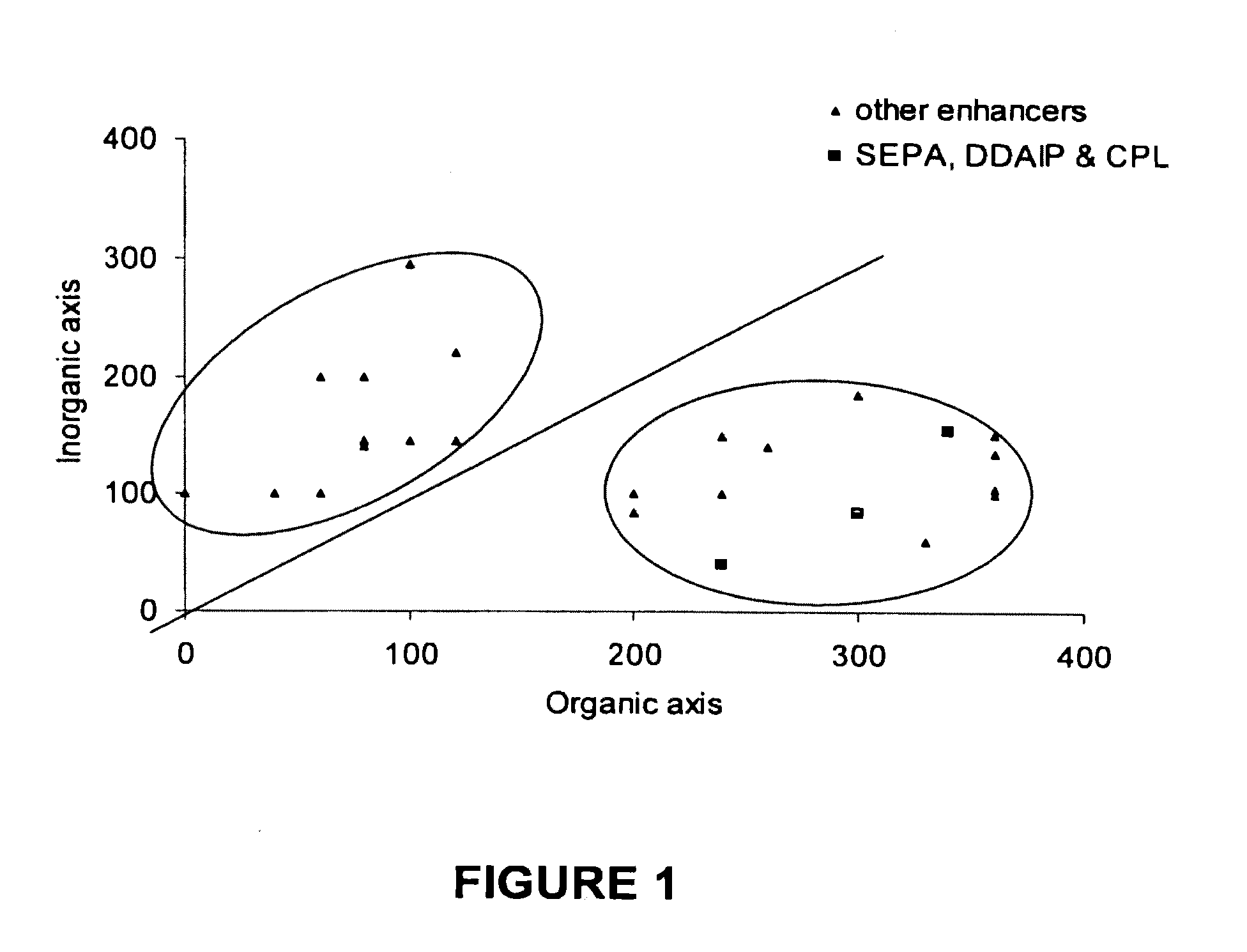
[0082]FIG. 1 shows the organic and inorganic values for typical penetration enhancers that can be used in accordance with the invention (determined by the method described by Fujita in “Production of organic compounds by a Conceptional Diagram”Chem. Pharm. Bull, Tokyo 1954 2:163). Area 1 being solvent based dermal penetration enhancers which are prone to irritate the skin or evaporate off it when using non-occlusive percutaneous or transdermal drug delivery systems. The preferred penetration enhancers are taken from the area 2 of the conceptional diagram (as originally proposed by Hori et al J. Pharm. Pharmacol 1990 42: 71-72). The preferred area spans an inorganic value of from about 0 to about 200 and an organic value of about 200 to about 400.
example 2
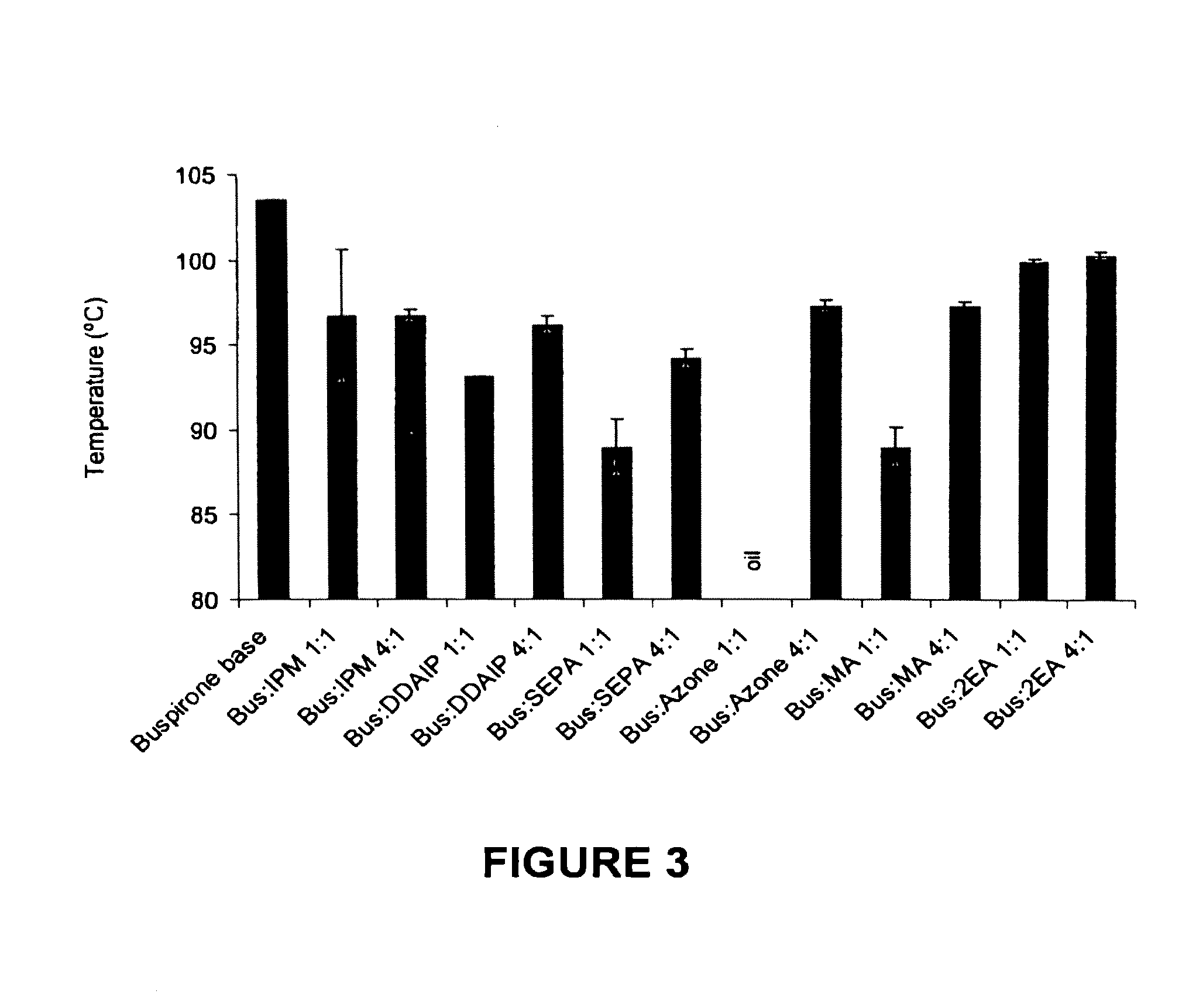
[0083]This example examines compositions of the invention formed by the combination of buspirone with a range of penetration enhancers having a range of organic and inorganic characteristics.
[0084]The physicochemical properties of buspirone are shown in the following table:
M. Wt (Da)LogPM. Pt (° C.)Buspirone385.512.63103.5
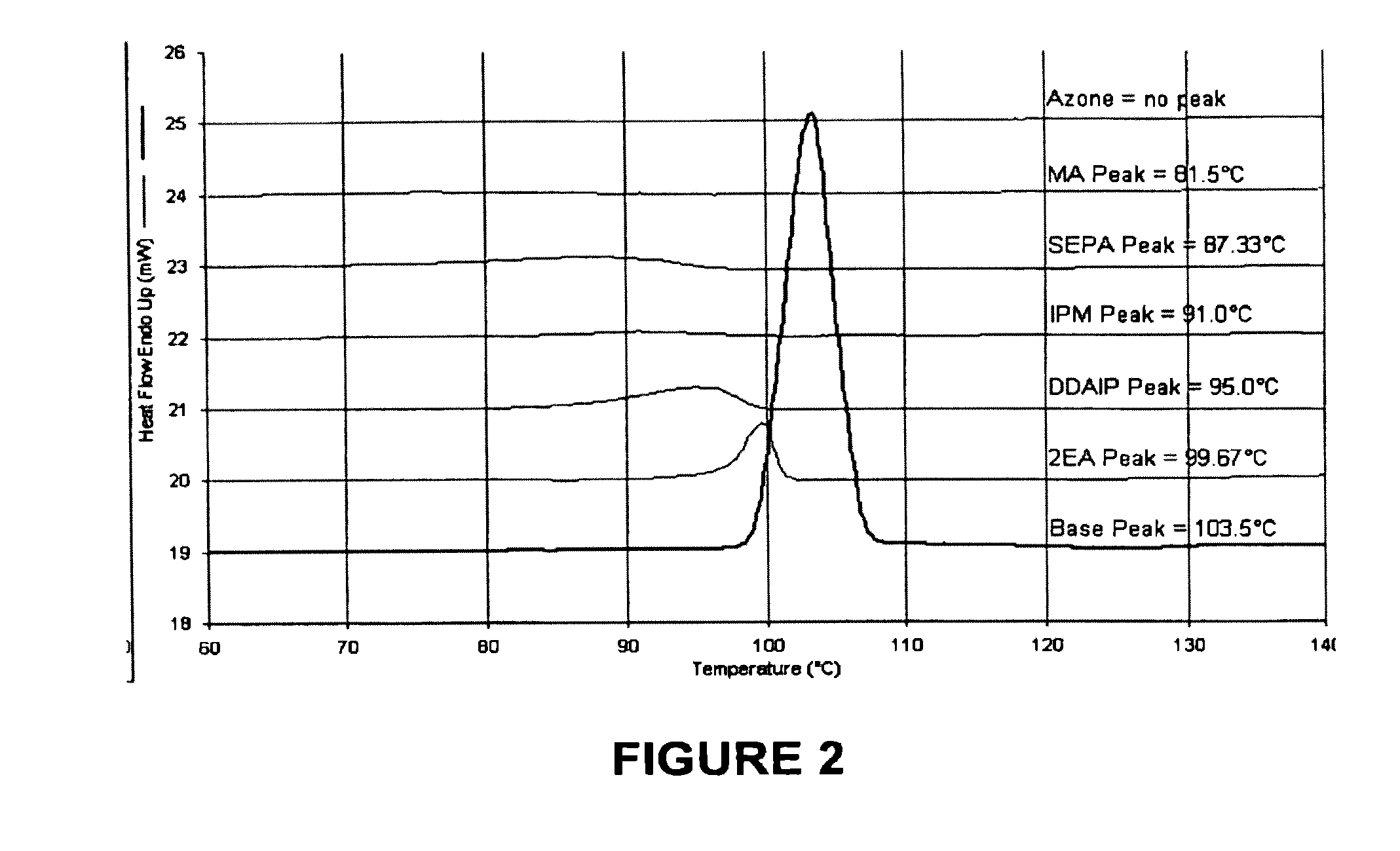
[0085]The penetration enhancers examined in this example were 2-n-nonyl, 1,3-dioxolane (SEPA), dodecyl 2-(N,N-dimethylamino)-propionate (DDAIP) and cylclopentadecanone (CPL).
[0086]Referring to FIG. 1 there is shown a plot of inorganic index against organic index for potential penetration enhancers. The organic and inorganic values are determined according to the procedure of Fujita A Chem. Pharm. Bull (Tokyo) 2:173 (1954). The compounds 2-n-nonyl, 1,3-dioxolane, dodecyl 2-(N,N-dimethylamino)-propionate (DDAIP) and cylclopentadecanone demonstrate a range of organic, inorganic index in Area 2 generally defining organic index between 0 and 200 and an organic index bet...
example 3
[0097]FIG. 5 shows the cumulative amount of buspirone diffused across human epidermis with time from a control containing buspirone in volatile liquid (95% ethanol) and a composition containing buspirone and octyl salicylate penetration enhancer in the same volatile liquid. Addition of the octyl salicylate to the transdermal spray formulation caused a significant marked increase in the amount of buspirone diffusing across the skin over 24 hours (p<0.05).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com