In-situ gel containing cyclosporin micelle as sustained-release ophthalmic drug delivery system
A technology of cyclosporine and micelles, which is applied in the field of pharmaceuticals, can solve the problems of increasing bioavailability, and achieve the effects of improving stability, increasing adhesion, and improving drug solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Determination of the medial concentration of the present invention
[0073] Table 2 lists samples containing 0.05% cyclosporine A:
[0074] Table 2. Sample formulation of cyclosporine A nano micelle solution
[0075]
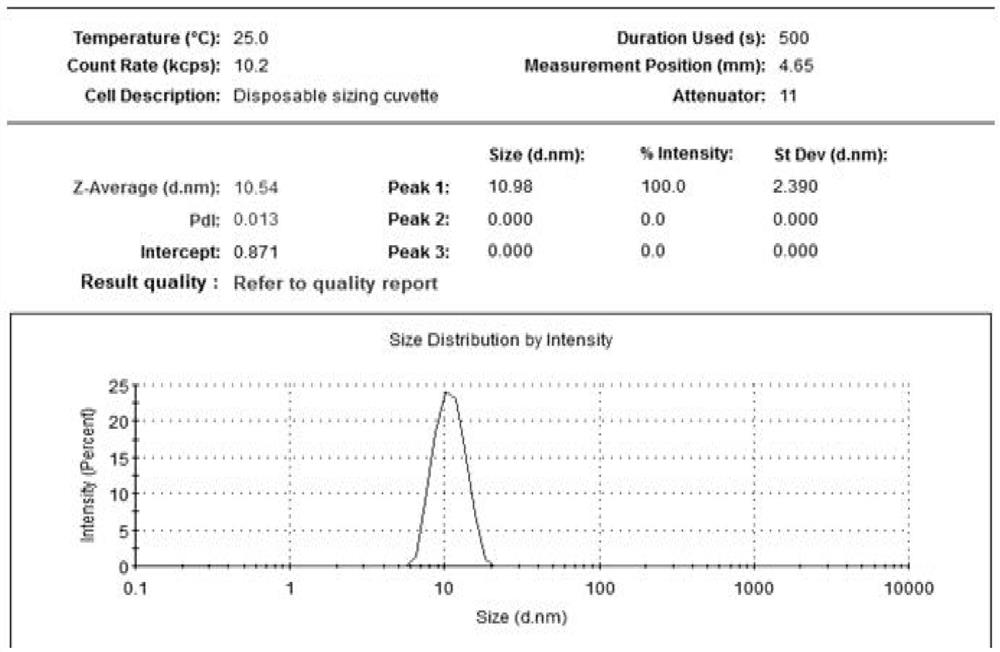
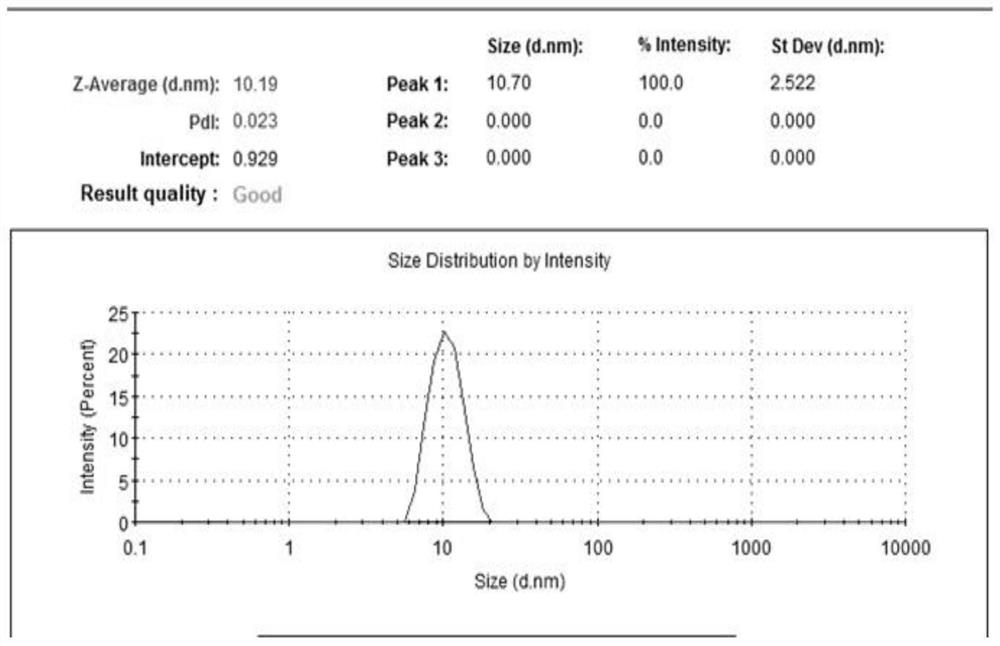
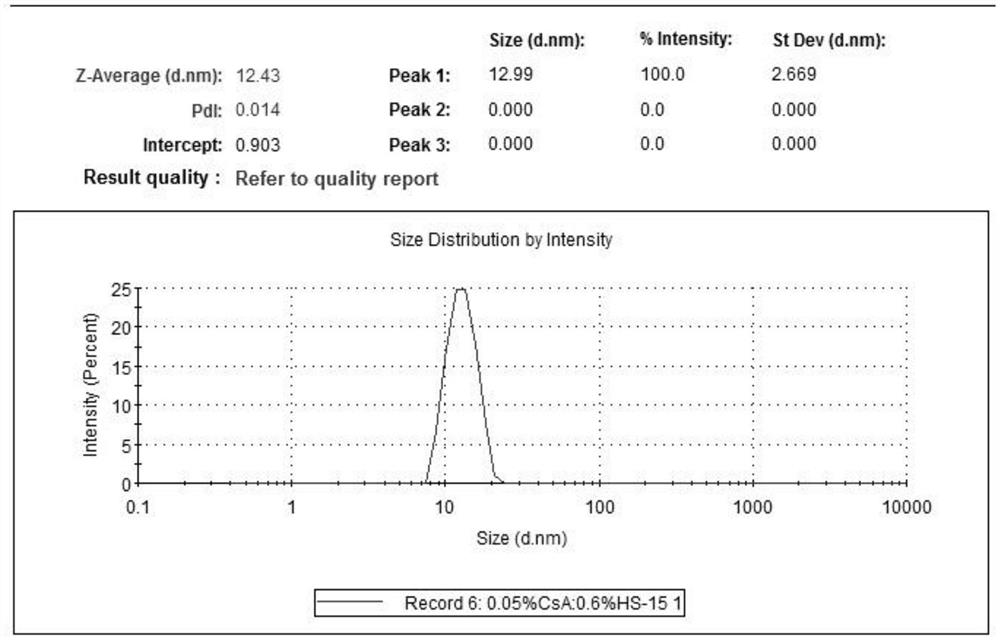
[0076] Granularity and distribution testing
[0077] The micellar particle size and distribution or multi-dispersion index (PDI) (Table 3) prepared by the above formulations were tested using a particle size analyzer. The result is shown Figure 1-8 And confirm that the micelle particle diameter in the sample 1-8 of the preparation and test is less than or
[0078] Table 3. Samples with Comparison of particle size of the middle nanometer micelles
[0079]
[0080]
Embodiment 2
[0081] Example 2: Determination of gel matrix concentration
[0082] Samples containing 0.05% cyclosporine A solution are listed in Table 4-7:
[0083] Table 4. Concentration of gel matrix finish
[0084]
[0085] Table 5. Concentration of gel matrix yellow original gum
[0086]
[0087] Table 6. The concentration of gel matrix kale gel
[0088]
[0089]
[0090] Table 7. Concentration of sodium gel matrix alginate
[0091]
[0092] Preparation method of gel solution
[0093] Accurately weighed a certain amount of sodium chloride, and add 85 grams of ultrapure water evenly. The solution was stirred until sodium chloride was completely dissolved, and then the above gel matrix was slowly added slowly with continuous stirring. This solution was placed in a 90 ° C water bath and stirred for 1 hour. The mixture was then cooled to room temperature. 0.05 g of cyclosporin A was weighed and the slowly added to a cooled solution under stirring. Add water to the final amount of 100...
Embodiment 3
[0109] Example 3: In situ gel of the cyclosporine micelles of the present invention.
[0110] The formulation of the micellar eye containing 0.05% cyclosporine A is as follows:
[0111] Cyclosporine A 0.05% by weight, 0.25% by weight of the deacetyl batch, 1% by weight of polyethylene glycol, 0.15% by weight of sodium chloride, 3.3% by weight of mannitol, hydroxybenzoic acid 0.02% by weight of the ester, an appropriate amount of a mito-dyric alkanol hydrochloric acid buffer and an injection of water to prepare an ophthalmic gel containing 0.05% cyclosporine micelles (Table 12).
[0112] Table 12. Composition of Example 3 Nano-micelle in situ gel
[0113] Component Percentage (wt%) Cyclosporine A 0.05 wt% Decetyl tilation 0.25 wt% Polyethylene glycol 20 hexadecion 1.0 wt% Sodium chloride 0.15 wt% Mannitol 3.3 wt% Hydroxybenzoate 0.02 wt% Aminobutyl alcohol hydrochloric acid buffer A certain amount of Water for Injecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com