Acetic acid copaxone microsphere and preparation method thereof
A technology of glatiramer acetate and microspheres, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, peptide/protein components, etc., can solve frequent problems, stabilize the condition, reduce irritation, and reduce the frequency of medication Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] Another aspect of the present invention provides a method for preparing glatiramer acetate microspheres, the method comprising:
[0060] (1) Add glatiramer acetate and protective agent to water for injection, stir until the drug is completely dissolved, and set aside.
[0061] (2) Weigh the polymer carrier and dissolve it in an organic solvent for later use.
[0062] (3) Mix the solutions prepared in steps (1) and (2) and add emulsifier to make W / O colostrum.
[0063] (4) Add the W / O colostrum in step (3) to the aqueous solution containing polyvinyl alcohol to make W / O / W double emulsion.
[0064] (5) Slowly stir the W / O / W double emulsion in step (4), evaporate the organic solvent, then filter, wash, centrifuge, freeze-dry to obtain glatiramer acetate microspheres.
[0065] in
[0066] The protective agent in step (1) is selected from one of mannitol, gelatin, hypromellose (HPMC, viscosity not greater than 10cp), sodium carboxymethylcellulose (NaCMC), polyvinylpyrrolido...
Embodiment
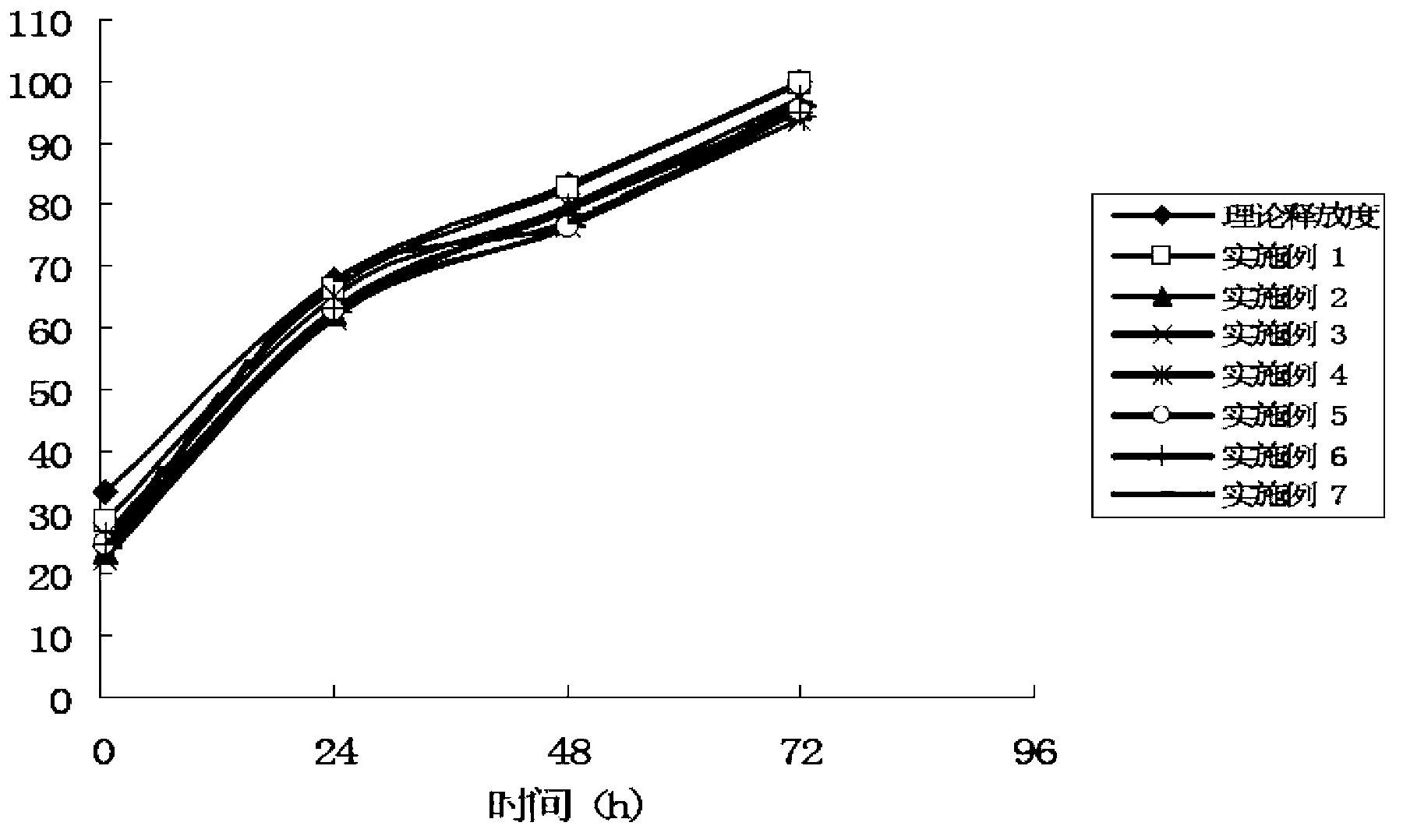
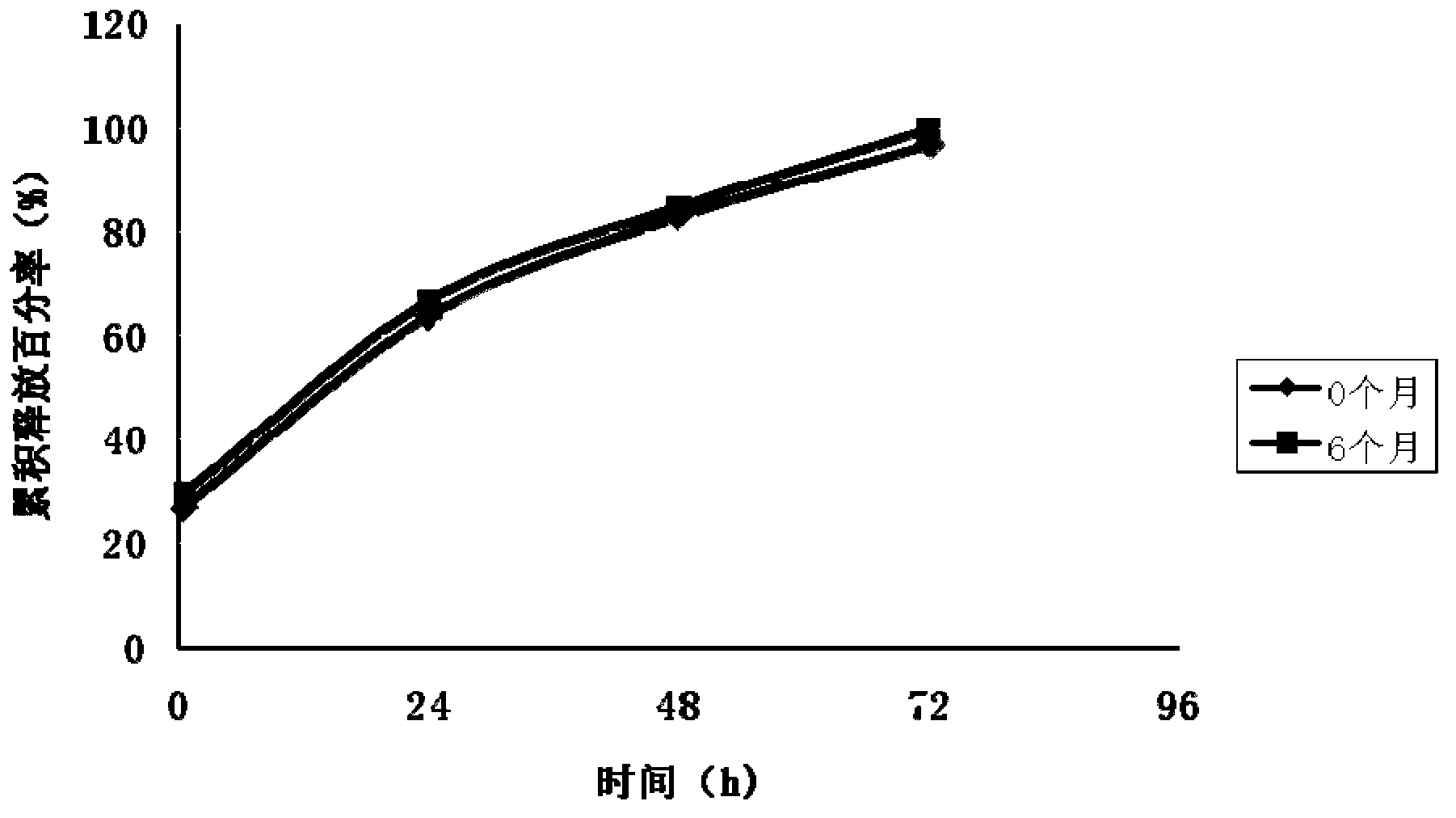
[0075] In order to further understand the present invention, the present invention will be described in detail below in conjunction with specific examples. It should be understood that the following examples are intended to illustrate rather than limit the present invention.
[0076] raw material
[0077] Glatiramer acetate (from Shenzhen Hanyu Pharmaceutical Co., Ltd., batch 20111109)
[0078] PLGA (purchased from Jinan Daigang Biotechnology Co., Ltd.)
[0079] Hypromellose (purchased from Anhui Shanhe Pharmaceutical Excipients Co., Ltd.)
[0080] Sodium carboxymethylcellulose (purchased from Anhui Shanhe Pharmaceutical Excipients Co., Ltd.)
[0081] Polyvinyl alcohol (purchased from Xi'an Zaolotang Pharmaceutical Group Rehabilitation Medicine Co., Ltd.)
[0082] Span 80 (purchased from Xi'an Yuelai Pharmaceutical Technology Co., Ltd.)
[0083] Polyvinylpyrrolidone (purchased from Shandong Liaocheng Ahua Pharmaceutical Co., Ltd.)
[0084] Mannitol (purchased from Qingdao...
preparation Embodiment 1
[0096] (1) Add 6 g of glatiramer acetate and 0.5 g of HPMC into 200 ml of water for injection, stir to dissolve the drug completely, and set aside.
[0097] (2) Weigh 70g of polymer carrier PLGA (the ratio of lactic acid to glycolic acid is 60:40) and dissolve it in 500ml of dichloromethane.
[0098] (3) Mix the prepared solutions of (1) and (2), add 0.2% (g / ml) Span 80 and stir at high speed with a homogenizer to make W / O colostrum.
[0099] (4) Inject W / O colostrum into 15L aqueous solution containing 0.3% (g / ml) polyvinyl alcohol (PVA), and make W / O / W double emulsion at 6000r / min.
[0100] (5) Slowly stir the above emulsion for 4 hours, evaporate the organic solvent to dry up the semi-solid microspheres, filter, wash with water 3 times, collect by centrifugation (3000r / min), disperse with 100ml of 6% mannitol solution, freeze-dry, 125.65 g of microsphere freeze-dried product was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com