Taxol long-circulating nanoparticle preparation and preparation method thereof
A paclitaxel and nanoparticle technology, which is applied in the field of preparation of the paclitaxel nanoparticle, can solve problems such as inability to complete large-scale production, stay in the laboratory, and immaturity, so as to maintain effective therapeutic concentration, reduce the number of medications, increase release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Implementation Example 1: Preparation of Forward Dropwise Long Circulation Nanoparticles
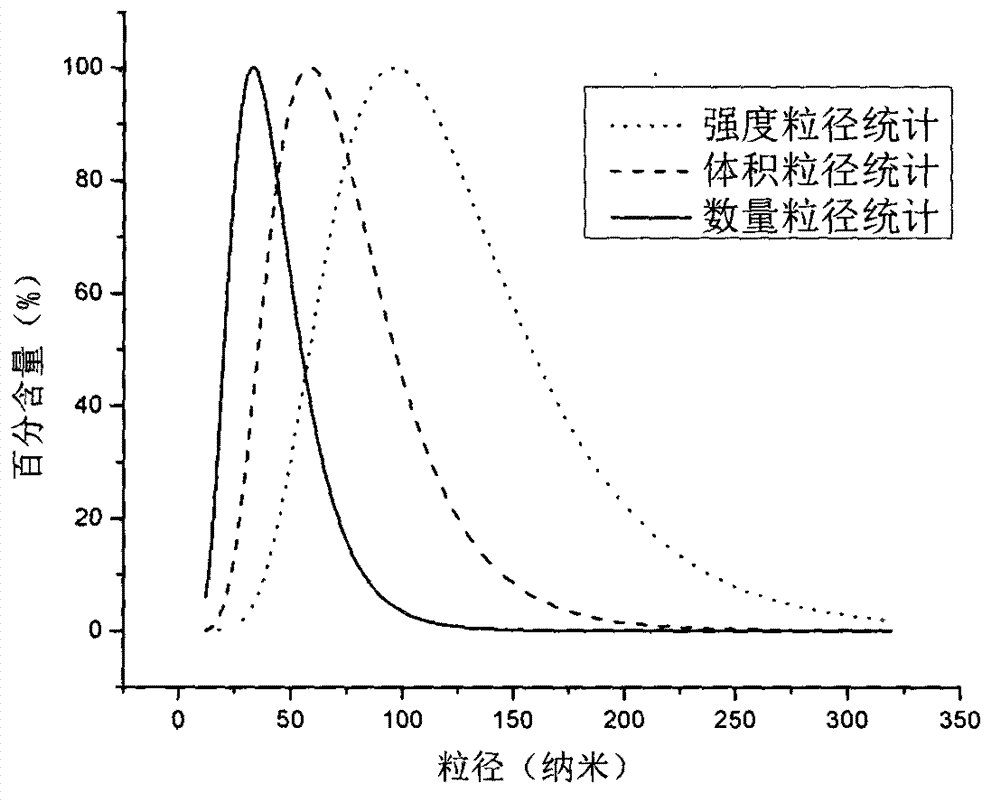
[0032] 40mg PLGA and 4mg paclitaxel were dissolved together in 10ml acetone solvent as oil phase, 120mg TPGS was dissolved in 50ml double distilled water to form water phase; the organic phase was dropped into the water phase at a speed of 1ml / min and stirred at a low speed of 300r / min to form a shallow phase. The blue nanoemulsion was reacted for 10 minutes, then transferred to a rotary evaporator, and treated for 30 minutes under vacuum rotary evaporation, and the acetone solution was removed to obtain long-circulation nanoparticles. The average particle size measured by the laser dynamic scattering instrument is 105.02±30nm, and the particle size distribution results are as follows: figure 1 shown. The encapsulation efficiency of nanoparticles is 80.2±4%, and the dispersion coefficient is 0.389.
Embodiment 2
[0033] Implementation Example 2: Preparation of Reverse Dropwise Long Circulation Nanoparticles
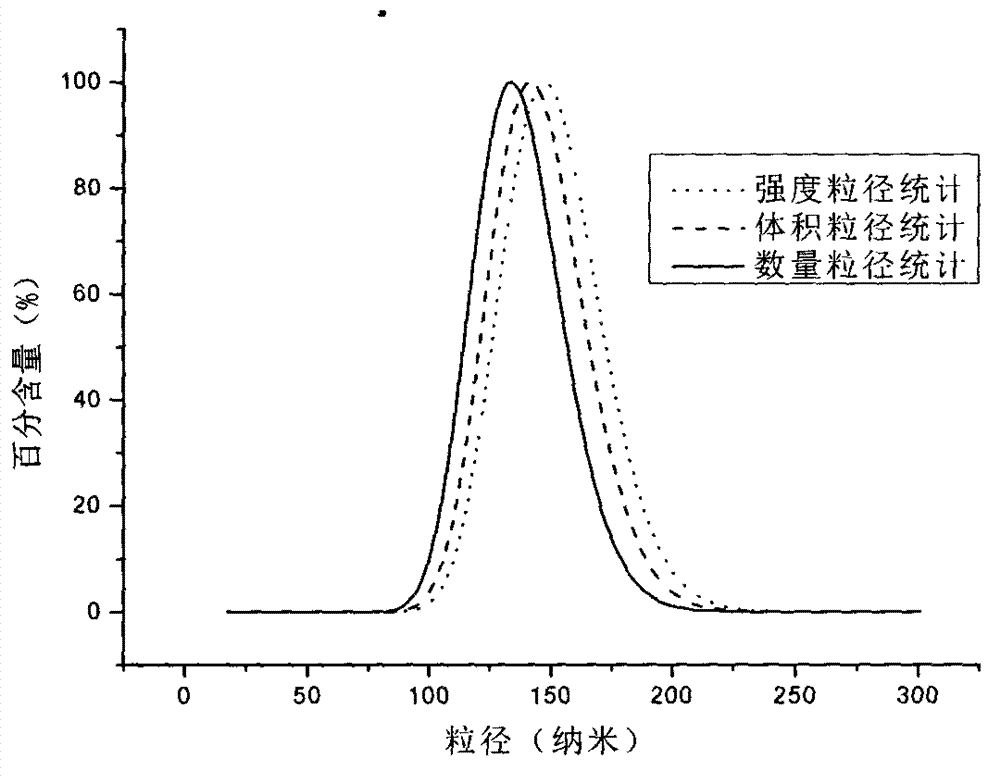
[0034] 40mg PLGA and 4mg paclitaxel were dissolved in 10ml of acetone solvent as an oil phase, 120mg TPGS was dissolved in 50ml double distilled water to form a water phase; the water phase was dropped into the organic phase at a speed of 5ml / min, and a shallow phase was formed under stirring at a low speed of 300r / min. The blue nano-emulsion, after reacting for 10 minutes, was transferred to a rotary evaporator, processed under vacuum rotary evaporation for 30 minutes, and the acetone solution was removed to obtain long-cycle nanoparticles. The result of diameter distribution is as figure 2 shown. The encapsulation efficiency of nanoparticles is 90.4±3%, and the dispersion coefficient is 0.042.
Embodiment 3
[0035] Implementation Example 3: Preparation of Reverse Dropwise Long Circulation Nanoparticles
[0036] 40mg PLGA and 8mg paclitaxel were dissolved together in 10ml acetone solvent as the oil phase, 120mg TPGS was dissolved in 50ml double-distilled water to form the water phase; the water phase was dropped into the organic phase at a speed of 5ml / min, and stirred at a low speed of 300r / min to form a shallow phase. The blue nanoemulsion, after reacting for 10 minutes, was transferred to a rotary evaporator, and treated for 30 minutes under vacuum rotary evaporation, and the acetone solution was removed to obtain long-cycle nanoparticles. The result of diameter distribution is as figure 2 shown. The encapsulation efficiency of nanoparticles is 84.4±3%, and the dispersion coefficient is 0.108.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com