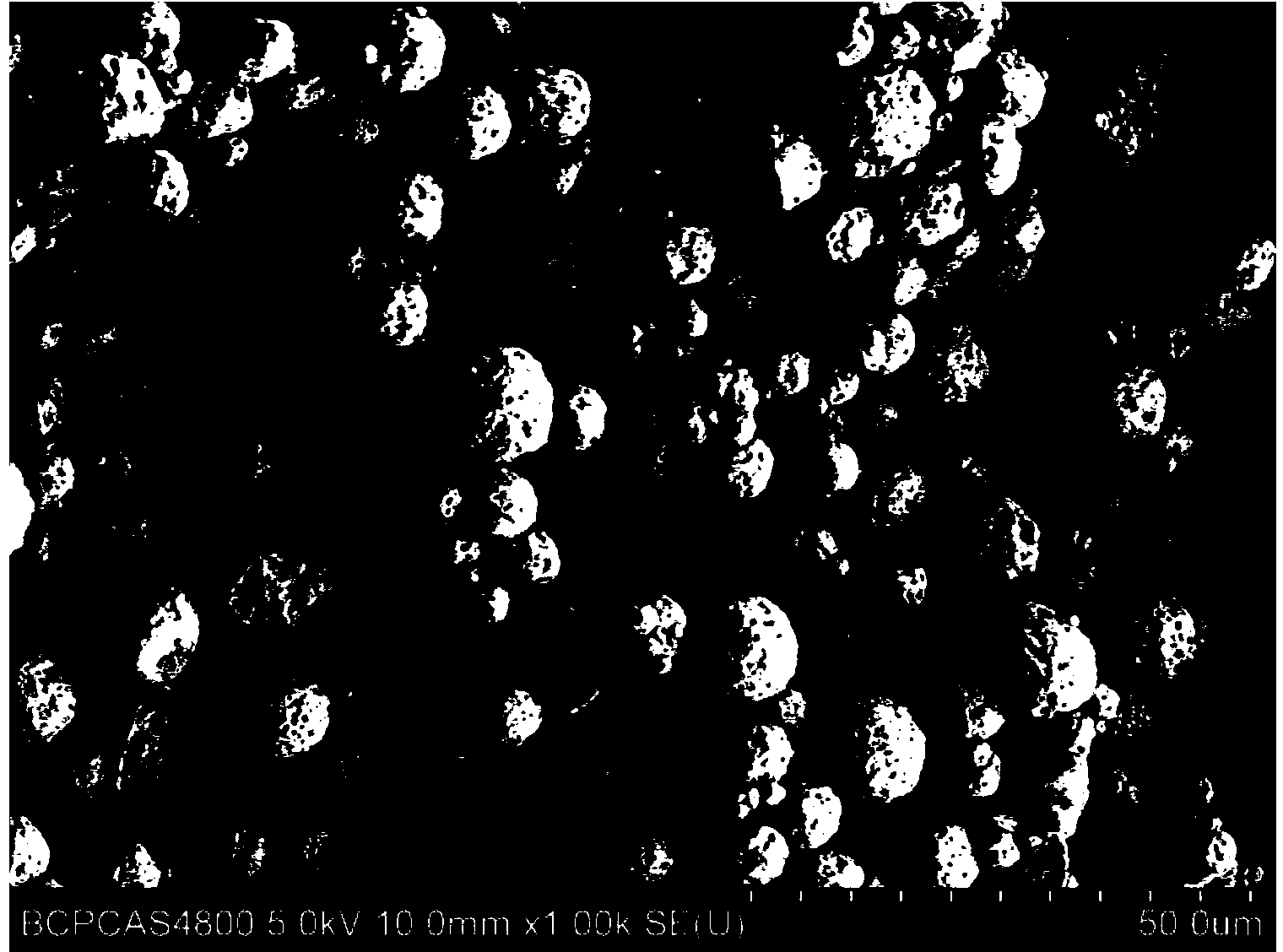Method for preparing acipimox-ethylcellulose sustained release micro-capsule
A technology of ethyl cellulose and acipimox, which is applied in the direction of pharmaceutical formulations, metabolic diseases, drug combinations, etc., can solve the problems of unsatisfactory life and drug application, and short half-life, so as to improve bioavailability, increase half-life, The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Use a drug pulverizer to pulverize amoxicillin to 80 mesh, and after ethylcellulose with a viscosity specification of 10CP is pulverized to 40 mesh, take amoxicillin drug powder and ethylcellulose powder, and its mass ratio is 3: 1. Slowly add ethyl cellulose to dichloromethane under stirring at a rotating speed of 400rmp to obtain solution a; add pulverized amoxicil drug powder to solution a under stirring at a rotating speed of 400rmp to obtain solution b ; The solution b was left to stand under stirring at a rotating speed of 400rmp, and the water with a volume ratio of 5:1 to dichloromethane was placed in a 30°C constant temperature water bath, and 1% emulsifier SDS was added while stirring to fully disperse the emulsifier. Obtain solution c; add solution b to solution c, continue to stir in a 30°C constant temperature water bath for 4 hours, then suction filter, wash and dry, and finally obtain microcapsules with good roundness and adhesion. Such as figure 1 As sh...
Embodiment 2
[0034] Use a drug pulverizer to pulverize amoxicillin to 80 mesh, and after ethylcellulose with a viscosity specification of 10CP is pulverized to 40 mesh, weigh amoxicillin drug powder and ethylcellulose powder, and its mass ratio is 2: 1. Slowly add ethyl cellulose to dichloromethane under stirring at a rotational speed of 250rmp to obtain solution a; add pulverized amoximus drug powder to solution a under stirring at a rotational speed of 250rmp to obtain solution b ; The solution b was left to stand under stirring at a rotating speed of 400rmp, and the water with a volume ratio of 4:1 to dichloromethane was placed in a 32°C constant temperature water bath, and 1% emulsifier SDS was added while stirring to fully disperse the emulsifier. Obtain solution c; add solution b to solution c, continue to stir in a 32°C constant temperature water bath for 3 hours, then suction filter, wash and dry, and finally obtain microcapsules with good roundness and adhesion.
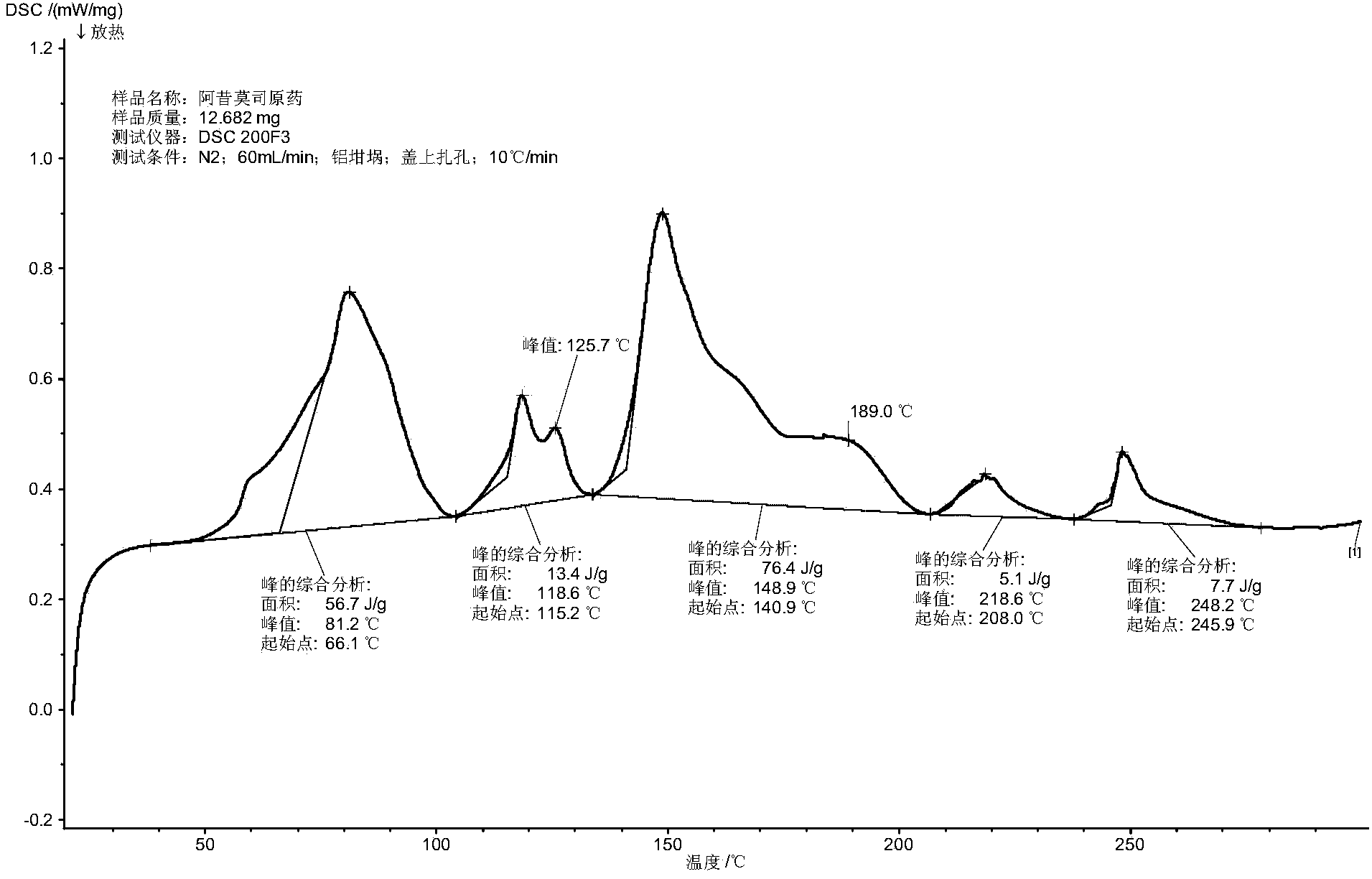
[0035] The relea...
Embodiment 3
[0037]Use a drug pulverizer to pulverize amoxix to 80 mesh, and after ethylcellulose with a viscosity specification of 10CP is pulverized to 40 mesh, weigh the amoxix drug powder and ethylcellulose powder, and the mass ratio is 1: 1. Slowly add ethyl cellulose to dichloromethane under stirring at a rotating speed of 300rmp to obtain solution a; add pulverized amoximus drug powder to solution a under stirring at rotating speed of 300rmp to obtain solution b The solution b was left to stand under stirring at a rotating speed of 300rmp, and the water with a volume ratio of 3:1 to dichloromethane was placed in a 35°C constant temperature water bath, and 1.5% emulsifier SDS was added while stirring to fully disperse the emulsifier. Obtain solution c; add solution b to solution c, continue to stir in a 35°C constant temperature water bath for 4 hours, then suction filter, wash and dry, and finally obtain microcapsules with good roundness and adhesion.
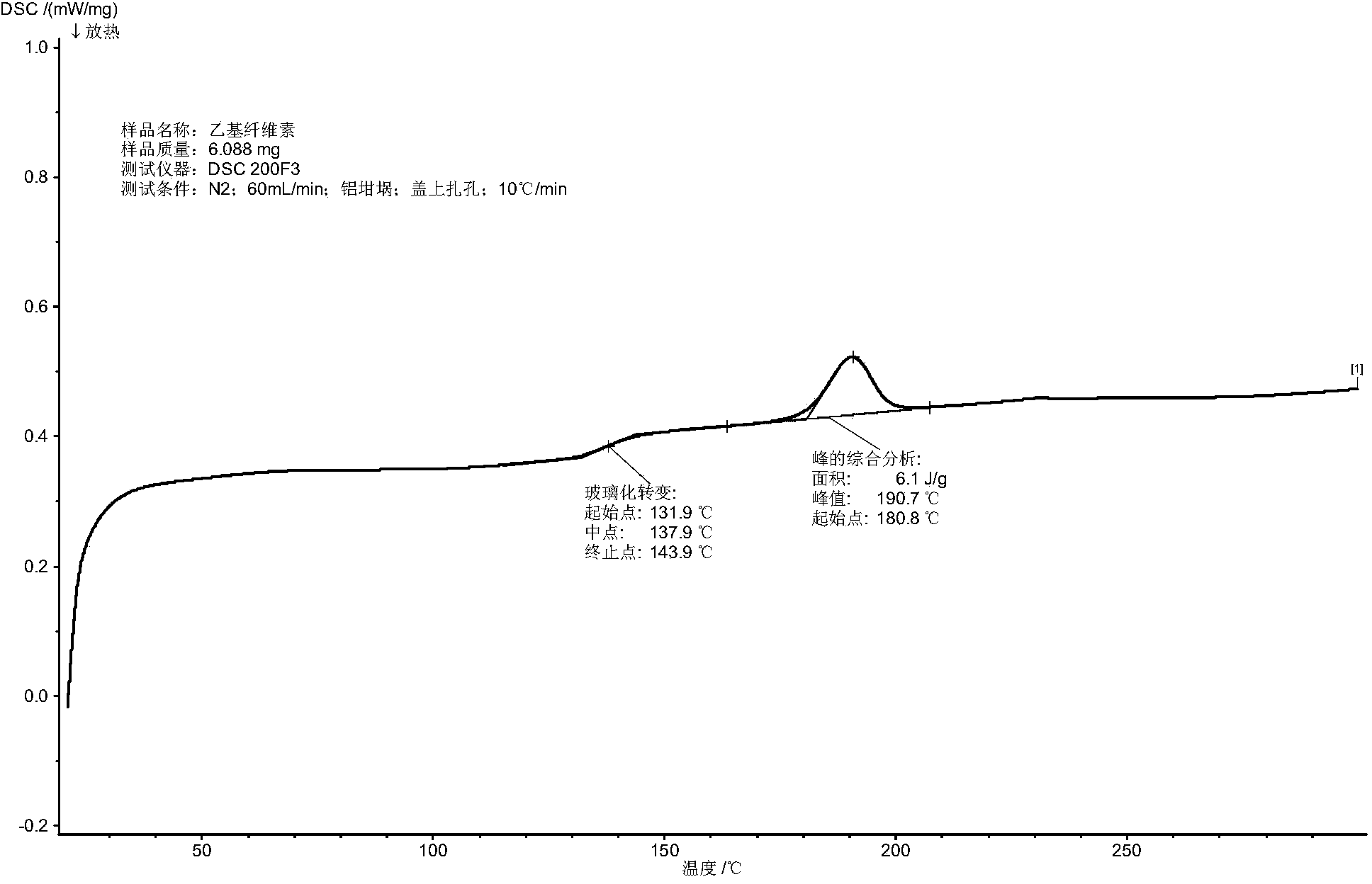
[0038] The release rate of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com