Patents
Literature
32results about How to "Reduce the number of crystallization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
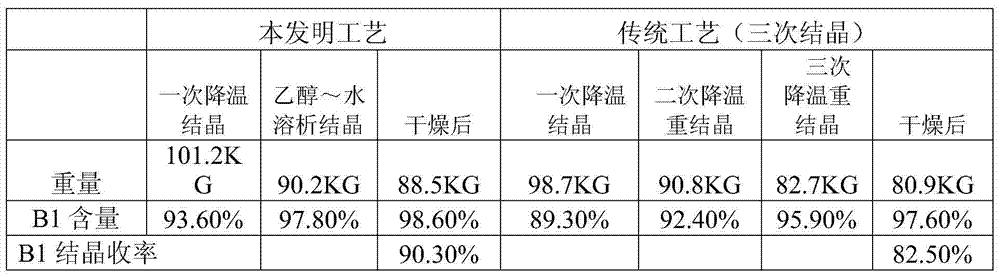
Crystallization method of abamectin Bla
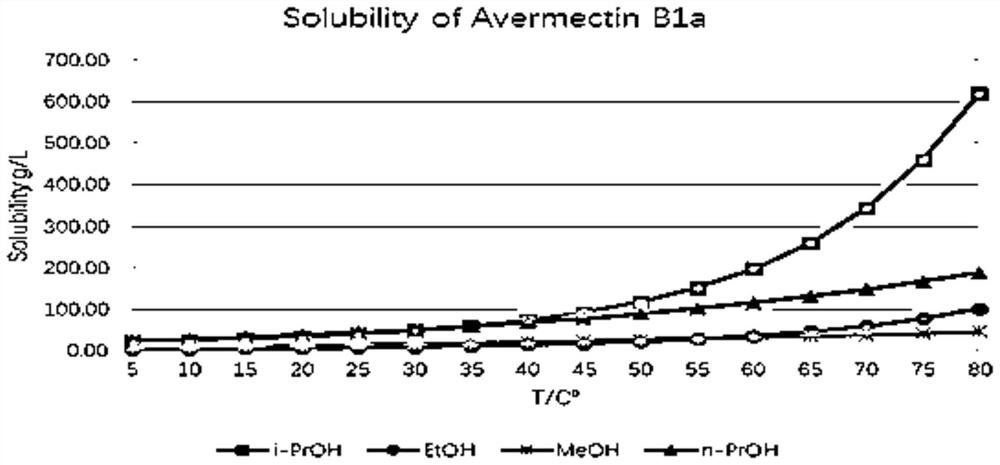
The present invention relates to a crystallization method of abamectin B 1a. Said method includes the following steps: using crystallization solvent n-butanol to stir and dissolve primary crude powder of abamectin B 1a at 75-100deg.C to saturation, filtering while the saturated solution is hot to obtain clear hot-saturated solution; slowly cooling said solution to that when the supersaturation degree is 1-3, adding crystal seeds, constant stirring for 20-60min, its stirring speed is 120-300rpm, and cooling to make crystallization, fitering crystal slurry or centrifugally-separating said crystal slurry, washing crystal and drying so as to obtain the invented abamectin B 1a.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Production process of high-purity abamectin fine powder
ActiveCN102617668AHigh purityImprove product qualitySugar derivativesSugar derivatives preparationAbamectinFermentation
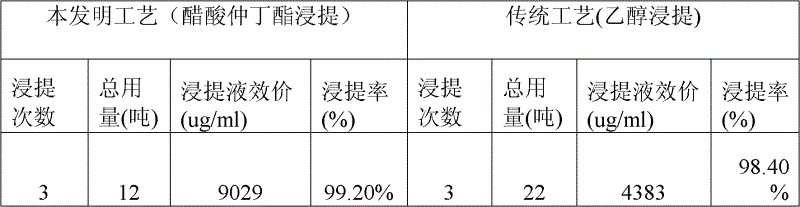
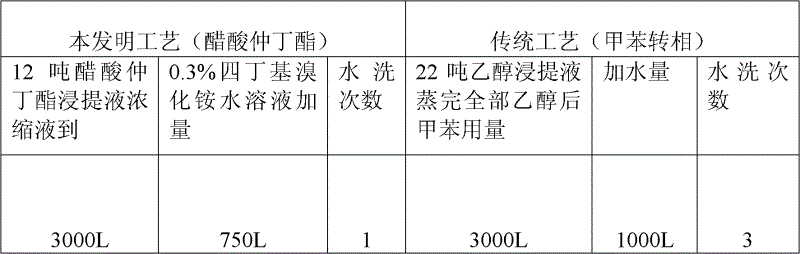
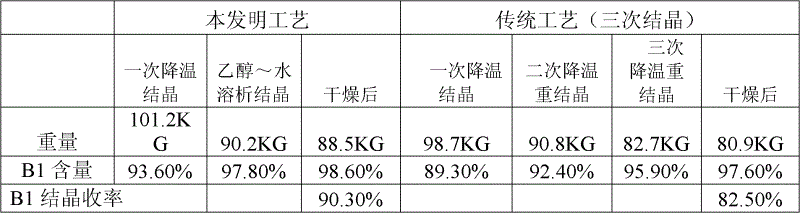
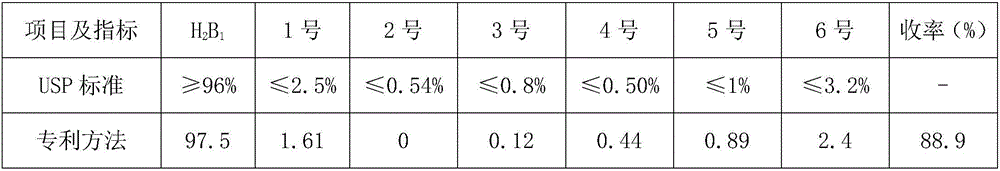
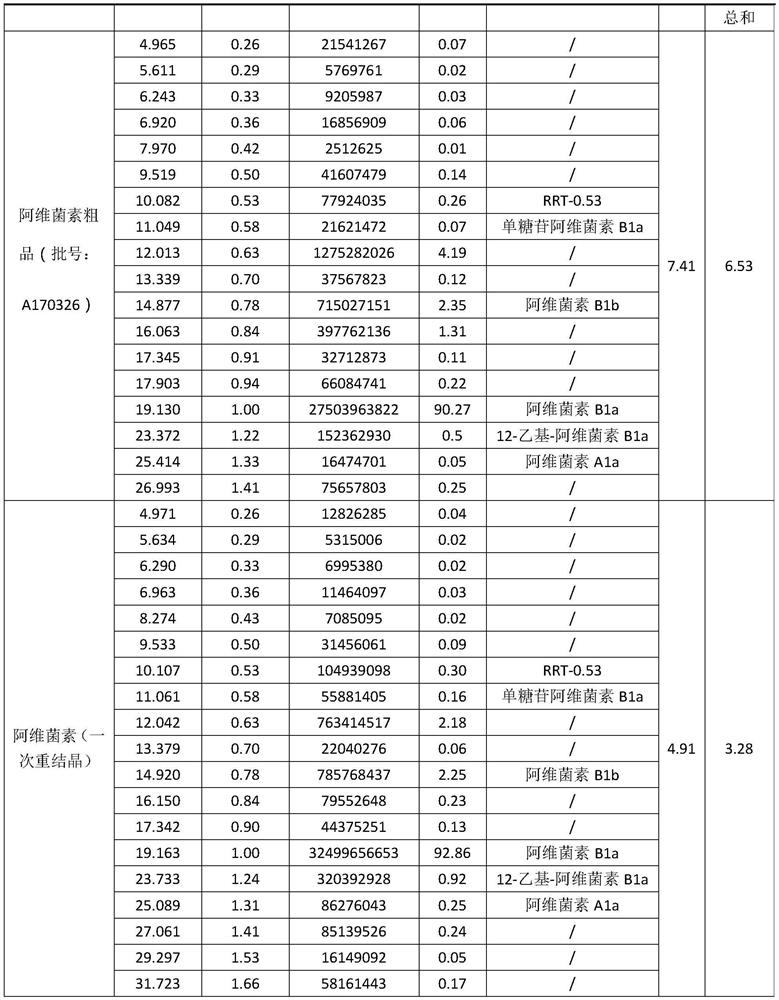
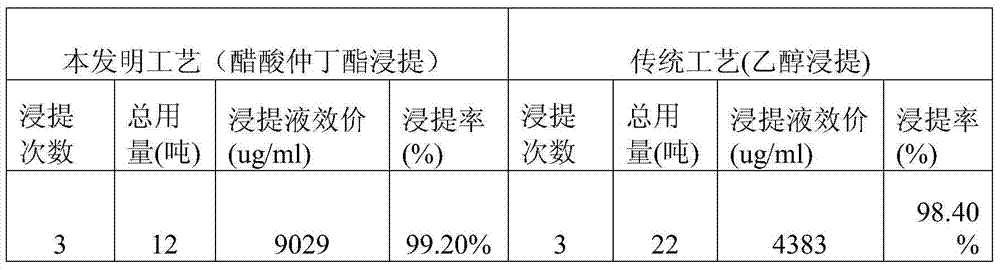
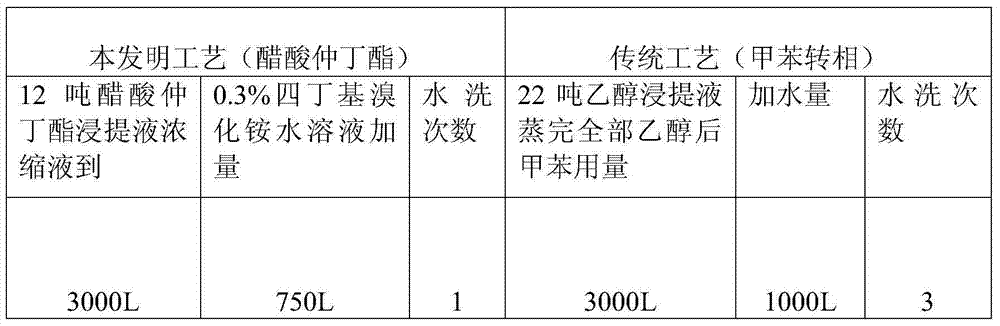
The invention relates to a production process of abamectin fine powder. The production process method comprises the steps: extracting fermentation liquid filter cake with sec-butyl acetate for three times; combining organic-ester-phase extracts, adding tetrabutyl ammonium bromide aqueous solution for washing, and filtering, concentrating filtrate, and cooling and crystallizing to obtain once crude product; and dissolving the once crude product with ethanol, filtering, naturally cooling to room temperature, adding water at a volume ratio of ethanol to water being (7 : 1)-(4 : 1) for dilution crystallization, washing and drying to obtain the abamectin fine powder product. By adopting the method, the abamectin crystal product with higher purity and yield can be obtained; and compared with the traditional methods, the method has the advantages that the crystallizing time is shorter, the repeated crystallizing frequency is reduced and the production cost is low, thereby being a low-cost and high-efficiency production process of the high-purity abamectin fine powder.
Owner:DAQING JEFENE BIO CHEM
Preparation method of griseofulvin
The invention discloses a preparation method of griseofulvin. The preparation method comprises the following steps of: A, heating griseofulvin fermentation liquor, adding calcium oxide to adjust the PH, carrying out solid-liquid separation by using a sheet frame, adding calcium oxide to a filter cake to granulate, and extracting by using acetone to obtain a griseofulvin acetone extracting solution; B, decoloring the griseofulvin acetone extracting solution by using an active carbon column to obtain a griseofulvin decoloring solution, feeding the decoloring solution into a concentration tank, and crystallizing by concentrating to obtain wet griseofulvin crystals; and C, washing the wet griseofulvin crystals once by using a dichloromethane and ethanol mixed solution while stirring, then washing the wet griseofulvin crystals once by using medicinal ethanol with the concentration of 95 percent while stirring, drying the crystals by using a vacuum drier, and carrying out superfine grinding on the dried crystals to obtain the griseofulvin product. Compared with a traditional preparation method, the preparation method disclosed by the invention has the advantages that the crystallization times in the process of preparing the griseofulvin in the prior art is reduced, the yield loss caused by recrystalization of griseofulvin is effectively avoided, the total yield of the griseofulvin is improved by 7-10 percent, and the production cost is greatly reduced.
Owner:内蒙古格林特制药有限责任公司
Preparation method of ivermectin
ActiveCN106188185ACrystallization EfficientPromote growthSugar derivativesSugar derivatives preparationPaint thinnerFiltration
The invention discloses a preparation method of ivermectin. The preparation method comprises the following steps: (1) preparing an ivermectin rough product; (2) dissolving the ivermectin rough product with a solvent, heating, stirring until the ivermectin rough product is completely dissolved, and maintaining the temperature; (3) adding purified water and formamide into a rough product solution, controlling the fed-batch flow velocity, and cooling, so as to obtain a diluent; (4) adding seed crystals into the diluent, and growing the crystals; (5) carrying out cooling crystallization on the diluent until reaching a certain temperature; and (6) carrying out suction filtration, washing and drying, so as to obtain high-purity ivermectin fine powder. The yield of the preparation method reaches above 85%, and a preparation process having the beneficial effects of simple process, stable yield and capability of producing high-purity ivermectin in a large scale is provided.
Owner:NORTH CHINA PHARMA GROUP AINO
Ganciclovir recovery method
ActiveCN101851239AHigh purityReduce the number of crystallizationOrganic chemistryRecovery methodActivated carbon
The invention relates to a ganciclovir recovery method, which comprises the steps that ganciclovir mother solution is concentrated, alkaline solution is added to adjust pH value to 10.0-13.5, temperature is kept to be constant at 20-60 DEG C for 10-60 minutes, active carbon is added and agitation is conducted for 1-3h, filtration is conducted, organic solution is added in filtrate to precipitate white crystals, filtration is conducted, filter cakes are dissolved in deionized water, hydrochloric acid is added to regulate pH value to 5-6, solid sediment is precipitated, after the solid sediment is heated and dissolved, the temperature of the solid sediment is slowly decreased by 0-30 DEG C to precipitate crystals, and the crystals are filtered and dried to obtain high-purity ganciclovir products. Compared with the prior art, the invention has the advantages of simple operation, high yield, low cost, no pollution and the like.
Owner:SHANGHAI YIWEI INDAL
Method for preparing high-purity stigmasterol from phytosterol oleate
The invention discloses a method for preparing high-purity stigmasterol from phytosterol oleate. The method comprises the following steps of taking phytosterol oleate as a raw material, and performingsubzero fractionation, saponification, solid-liquid separation and two times of crystallization and purification to obtain a stigmasterol product with purity equal to 95% or more. Compared with the prior art, in the method, the phytosterol oleate is taken as the raw material to prepare the high-purity stigmasterol, the method has fewer crystal number of times and is simple in operation steps, thesolvent used for crystallization and purification can be recycled, making full use of resources is realized, and the production cost is lowered.
Owner:XIAN HEALTHFUL BIOTECH
Catalyst for producing polylactic resin and its prodn. process
InactiveCN1831027AIncreased crystallization yield and operating costsReduced cleanliness requirementsReaction timingChemistry
The invention relates to an analyzer producing the polylactic and the producing process of producing the polylactic adopting the analyzer , characterized in that it is composed by the composite containing the strontium and the aluminium namely the compound system of the bimetal o-oxo bridge alkoxylating compound and the benznene sulfonic acid; the mol content of the said bimetal o-oxo bridge alkoxylating compound is 40-90 percent; the process of producing the polylactic adopting the analyzer : (1)the lactide of the melting range of 92-108 degree is embedded in the reactor and chilled, heated up; (2) it is vacuumized to dewater fully on the N2 condition; (3) the definite proportion of the composite catalyst mixing agent of the Sr and aluminium are added and mixed equably; (4) the air of the reactor is displaced by the highly pure nitrogen gas in order to keep the positive pressure; (5) It is mixed and heated up and the reaction can be finished within 4-16 hours. Because of adopting the analyzer to the production, the puzzle of the high purify requirement of the gathering monocase lactide needed by the existing ring-opening polymerization; so the reaction time is shortened, the producing rate is improved and the producing cost is reduced.
Owner:RUGAO FIBERGLASS FACTORY
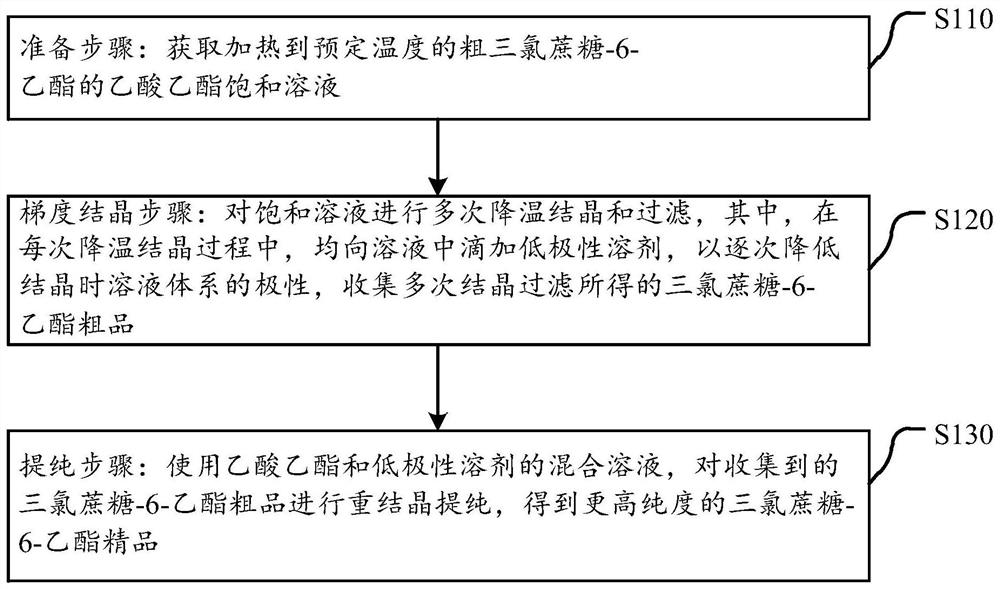
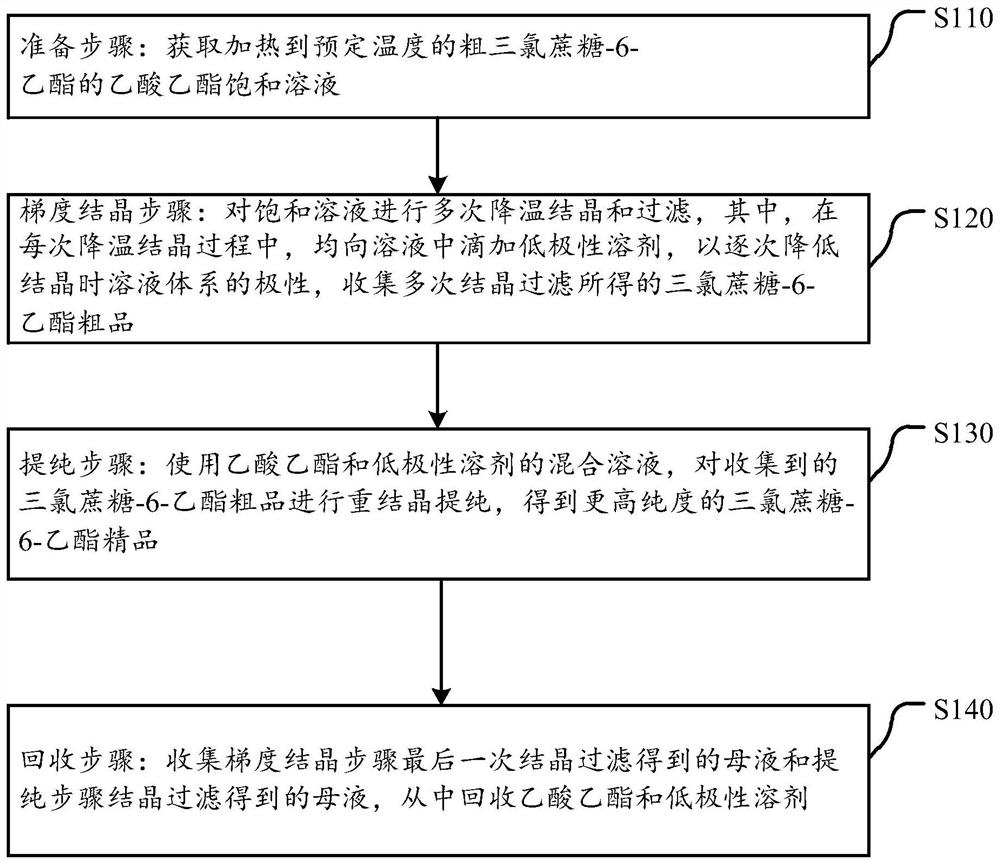
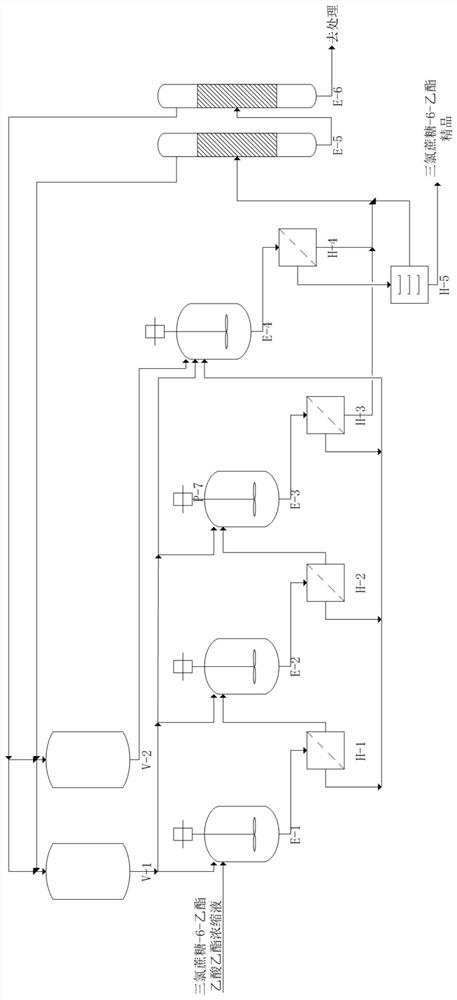
Purification method of sucralose-6-ethyl ester
ActiveCN112638924AHigh single-pass crystallization yield and efficiencyGuaranteed purityEsterified saccharide compoundsSugar derivativesSolventEthyl acetate
The invention discloses a purification method of sucralose-6-ethyl ester. The purification method comprises the following steps: a preparation step: obtaining an ethyl acetate saturated solution of crude sucralose-6-ethyl ester heated to a preset temperature; a gradient crystallization step: cooling crystallizing and filtering the saturated solution for multiple times, dropwise adding a low-polarity solvent into the solution in each cooling crystallizing process to gradually reduce the polarity of the solution system during crystallizing, and collecting a sucralose-6-ethyl ester crude product obtained by multiple times of crystallization and filtration; and a purification step: recrystallizing and purifying the collected sucralose-6-ethyl ester crude product by using a mixed solution of ethyl acetate and a low-polarity solvent to obtain a sucralose-6-ethyl ester fine product with higher purity. On the basis of cooling crystallization of sucralose-6-ethyl ester by using ethyl acetate as an initial solvent, the polarity of the mixed solvent is reduced by gradually adding a low-polarity solvent, so that the effectiveness of impurity separation in each gradient stage is improved, the product yield is high, and the product quality is good.
Owner:ANHUI JINGHE IND
Method for treating solid wastes of fluoroethylene carbonate ester
ActiveCN110775990AEffective protectionHeating evenlyAlkali metal chloridesAlkali metal halide purificationInfraredCarbonate ester
The invention discloses a method for treating solid wastes of fluoroethylene carbonate ester. The method comprises the following steps: 1) heating and decomposing fluoroethylene carbonate ester drogsin infrared equipment at a certain temperature; 2) adding a proper amount of hot water with a certain amount of potassium hydroxide into the decomposed drogs, performing stirring dissolution, performing concentration, and performing primary crystallization in a normal-temperature environment; 3) draining off water of a primary crystal, adding the primary crystal into clean water, performing heating to a certain temperature, performing stirring dissolution, performing concentration, and performing secondary crystallization in a normal-temperature environment; and 4) drying a secondary crystal in the infrared equipment at a certain temperature, so as to obtain a byproduct potassium chloride. By adopting the method for treating solid wastes of fluoroethylene carbonate ester, the infrared equipment is adopted for preheating, uniform heating can be implemented, the heating speed is rapid, organic matter in the solid drogs can be removed, caking is not easily caused, in addition, crystallization times can be reduced, treatment steps can be reduced, and the treatment cost can be correspondingly reduced.
Owner:江苏瀚康新材料有限公司
Seed crystal addition and atmosphere protection device for preparing high-purity gallium through crystallization method
InactiveCN105132718ASimplify the addition processShorten the production cycleProcess efficiency improvementEngineeringContact mode
The invention discloses a seed crystal addition and atmosphere protection device for preparing high-purity gallium through the crystallization method. A threaded hole is formed in the center of an end cover, and a lifting rod is a threaded rod and installed on the end cover through the threaded hole in the center of the end cover. The end cover is connected and matched with the top end of a crystallizer in a sealed mode. A support is connected to the lower end of the lifting rod, the lower ends of vertical through pipes are fixedly connected to the support, and the upper ends of the vertical through pipes are located above the end cover through through holes formed in the end cover. Supporting feet are arranged at the outer end of the support and matched with the inner wall of the crystallizer in a contact mode. An air inlet hole way is formed between the end cover and the support. The lifting rod can be solid or hollow; when the lifting rod is hollow, the central hole way of the lifting rod is the air inlet hole way. A handle is arranged at the upper end of the lifting rod. The supporting feet are uniformly arranged. The end cover and the crystallizer are matched in shape. According to the invention, the seed crystal addition process can be effectively simplified, the production cycle can be shortened, the influence of the production environment atmosphere to the purification process can be effectively avoided, and the production cost can be saved.
Owner:NORTHEASTERN UNIV
Purification method of puerarin
InactiveCN108586440AReduce the number of crystallizationReduce lossesOrganic chemistryChemistryPuerarin
The invention relates to a purification method of puerarin and belongs to the technical field of traditional Chinese medicine extraction. The purification method comprises the steps of 1, condensing in vacuum, puerarin elution under the temperature of 60-100 DEG C and vacuum degree of 0.04-0.09 MPa until puerarin concentration is 0.15 kg / L; 2, adding 2 L of the condensed puerarin elution into 0.2-1.0 kg of alumina, and adsorbing by stirring for 10-30 min; 3, filtering, and washing the alumina to obtain puerarin solution; 4, subjecting the puerarin solution of step 3 to septuple crystallizationto obtain puerarin having the content of greater than 99%. The purification method has the advantages that the purification method is scientific and reasonable and is simple and feasible, the purification method of puerarin is greatly simplified, production cost is reduced, and high-purity puerarin is obtained.
Owner:REYOUNG PHARMA
Preparation method for D-mannose crystals
InactiveCN103497221AImprove crystallization yieldShort processSugar derivativesSugar derivatives preparationIon exchangeEvaporation
The invention provides a preparation method for D-mannose crystals. The method includes adding activated carbon into mannose slurry to perform heating and discoloration, then performing desalination and purification, performing evaporation and concentration, and soaking in absolute ethyl alcohol for purification and drying to obtain high-purity crystal D-mannose. By the aid of the method that the activated carbon is used for discoloration and electrodialysis desalination, evaporation and concentration are performed until the slurry is almost oven dry and the absolute ethyl alcohol is used for soaking the obtained crystals, the problems of viscous mother liquid, small crystals, low yield and the like in the existing D-mannose crystallization process are solved. On the basis of the traditional technology, an advanced electrodialysis desalination technique replaces ion exchange, crystallization time ranges from 20 hours to 30 hours, a technological process is shortened, and energy conservation and consumption reduction are achieved; crystallization times are decreased, and crystallization yield of the mannose crystals is increased.
Owner:山东福田科技集团有限公司
Method for purifying sucralose-6-ethyl ester
ActiveCN113366006AHigh purityImprove qualityEsterified saccharide compoundsSugar derivativesAlkaneSucrose
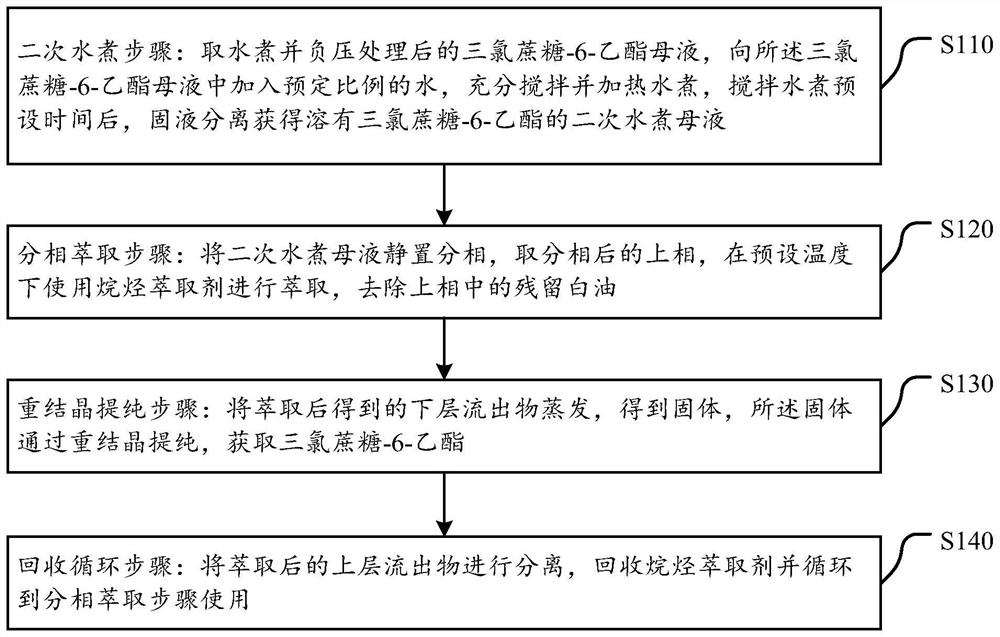
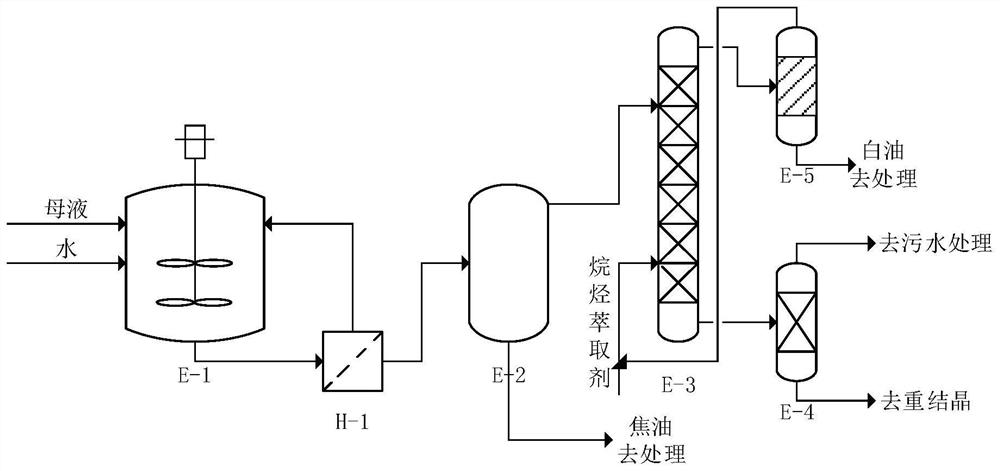
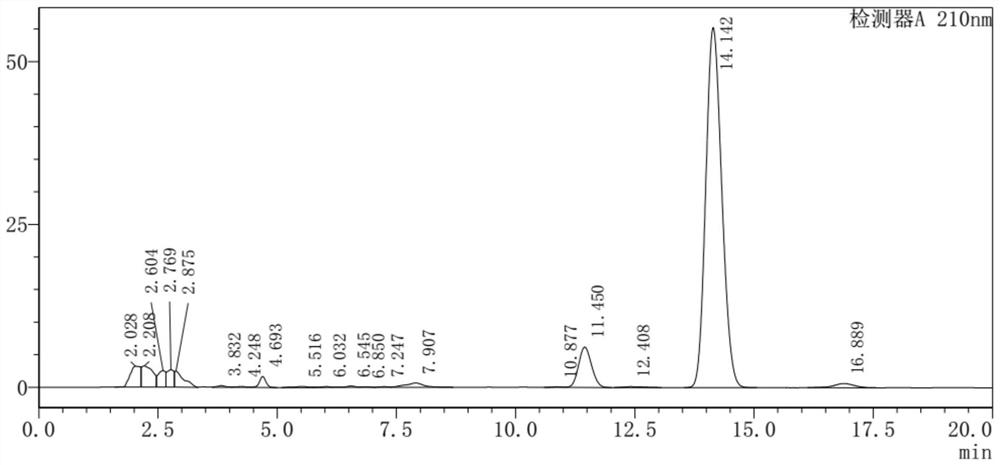
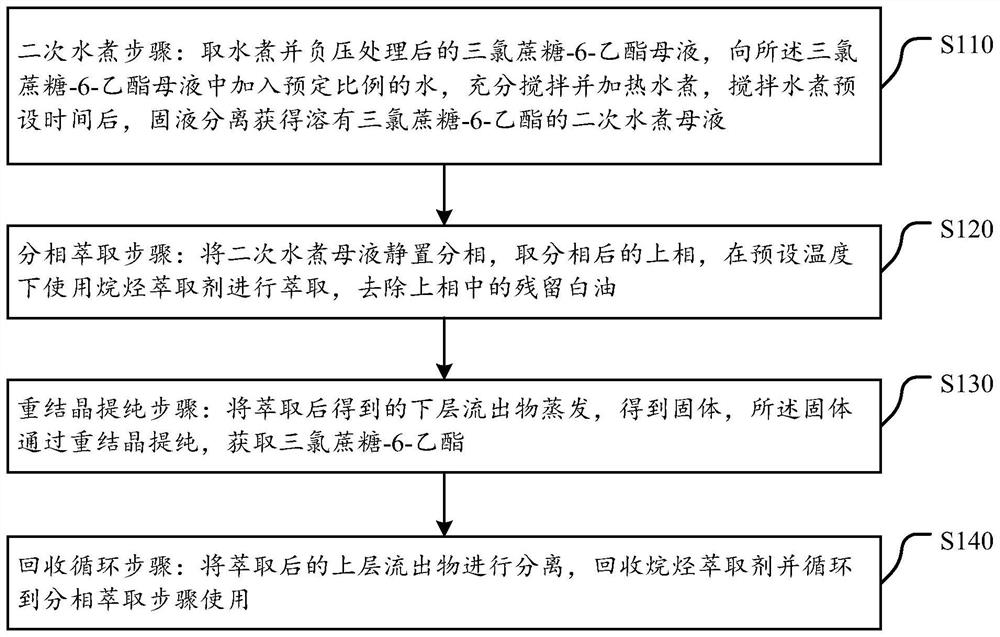
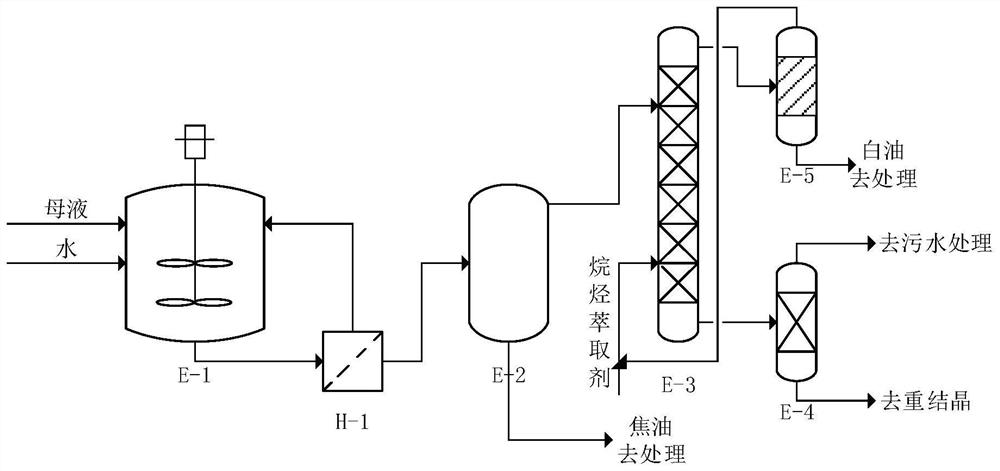
The invention discloses a sucralose-6-ethyl ester purification method which comprises the following steps: a secondary water boiling step: taking sucralose-6-ethyl ester mother liquor subjected to water boiling and negative pressure treatment, adding a preset proportion of water into the sucralose-6-ethyl ester mother liquor, fully stirring and heating for water boiling, and after stirring and water boiling for a preset time, filtering to obtain a filtrate; carrying out solid-liquid separation to obtain a secondary water boiling mother solution in which sucralose-6-ethyl ester is dissolved; a phase separation extraction step: standing the secondary water boiling mother liquor for phase separation, taking an upper phase after phase separation, extracting by using an alkane extraction agent at a preset temperature, and removing residual white oil in the upper phase; a recrystallization purification step: evaporating the lower effluent obtained after extraction to obtain a solid, and purifying the solid through recrystallization to obtain sucralose-6-ethyl ester; and a recycling step: separating the extracted upper-layer effluent, recycling the alkane extraction agent, and recycling the alkane extraction agent to the split-phase extraction step for use. The method is simple, efficient and low in cost, trace white oil can be effectively removed, and the purity of sucralose-6-ethyl ester is improved.
Owner:ANHUI JINGHE IND
Synthetic process of acipimox
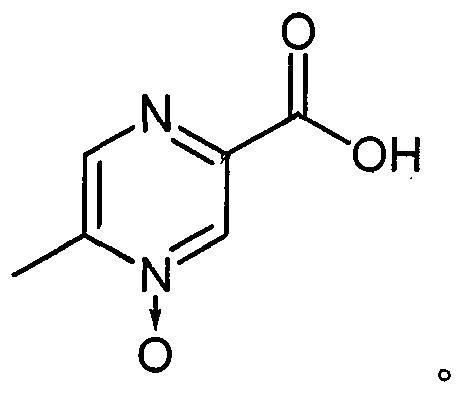
InactiveCN105218464BReduce the number of crystallizationAvoid recrystallization operationsOrganic chemistryOXALIC ACID DIHYDRATEAdjuvant
Owner:SICHUAN YIMING PHARMA CO LTD
A kind of preparation method of ivermectin
ActiveCN106188185BCrystallization EfficientPromote growthSugar derivativesSugar derivatives preparationFiltrationDiluent
The invention discloses a preparation method of ivermectin, comprising the following steps: 1) preparing crude ivermectin; 2) dissolving the crude ivermectin in a solvent, heating and stirring until completely dissolved, and keeping warm; 3) ) adding purified water and formamide to the crude product solution and controlling the flow rate, cooling down to obtain a diluted solution; 4) adding seed crystals to the diluted solution to grow crystals; 5) cooling and crystallizing the diluted solution to a certain temperature; 6) High-purity ivermectin fine powder can be obtained through suction filtration, washing and drying. The yield of the preparation method of the present invention reaches more than 85%. The present invention provides a preparation process with simple process, stable yield and large-scale production of high-purity ivermectin.
Owner:NORTH CHINA PHARMA GROUP AINO
Camptothecin separation and purification method
ActiveCN105061447AAdvantages of separation and purification methodsReduce contentOrganic chemistryChromatographic separationPurification methods
The purpose of the present invention is to disclose a camptothecin separation and purification method, particularly a technology for preparing high-purity camptothecin by using diatomaceous earth filtration, membrane separation technology, macroporous adsorption resin column chromatography adsorption and chromatography column preparation integration purification. With the method of the present invention, the problems that the selectivity of extraction and adsorption on the target compound is low, the purity of the prepared camptothecin is not high, and the multiple extraction-recrystallization way has disadvantages of high organic solvent consumption, labor consuming, time consuming, poor economy, high industrial production and serious environmental pollution are solved.
Owner:HUNAN XINLI BIOLOGICAL SCI & TECH +1
Preparation method of argatroban intermediate
PendingCN112538043AAchieve separationQuality improvementOrganic chemistryEngineeringCombinatorial chemistry
The invention discloses a preparation method of an argatroban intermediate, belonging to medicine preparation. The invention particularly relates to the field of preparation of medicine intermediates,and especially relates to an enzyme resolution preparation method for an important argatroban intermediate, namely ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate L-tartrate. The invention aims at overcoming the defects of excessive crystallization times and low yield of the existing process, and provides the preparation method, which is simple in research, design and operation, good in selectivity, high in yield and easier for industrial production, for the argatroban intermediate ethyl (2R,4R)-4-methyl-2-piperidinecarboxylate L-tartrate. The preparation method is a brand-new preparation method.
Owner:安庆恩聚生物医药科技有限公司
A kind of separation and purification method of camptothecin
ActiveCN105061447BReduce contentReduce consumptionOrganic chemistryChromatographic separationPurification methods
The purpose of the present invention is to disclose a camptothecin separation and purification method, particularly a technology for preparing high-purity camptothecin by using diatomaceous earth filtration, membrane separation technology, macroporous adsorption resin column chromatography adsorption and chromatography column preparation integration purification. With the method of the present invention, the problems that the selectivity of extraction and adsorption on the target compound is low, the purity of the prepared camptothecin is not high, and the multiple extraction-recrystallization way has disadvantages of high organic solvent consumption, labor consuming, time consuming, poor economy, high industrial production and serious environmental pollution are solved.
Owner:HUNAN XINLI BIOLOGICAL SCI & TECH +1
A method for separating cholesterol and 24-dehydrocholesterol by complex crystallization
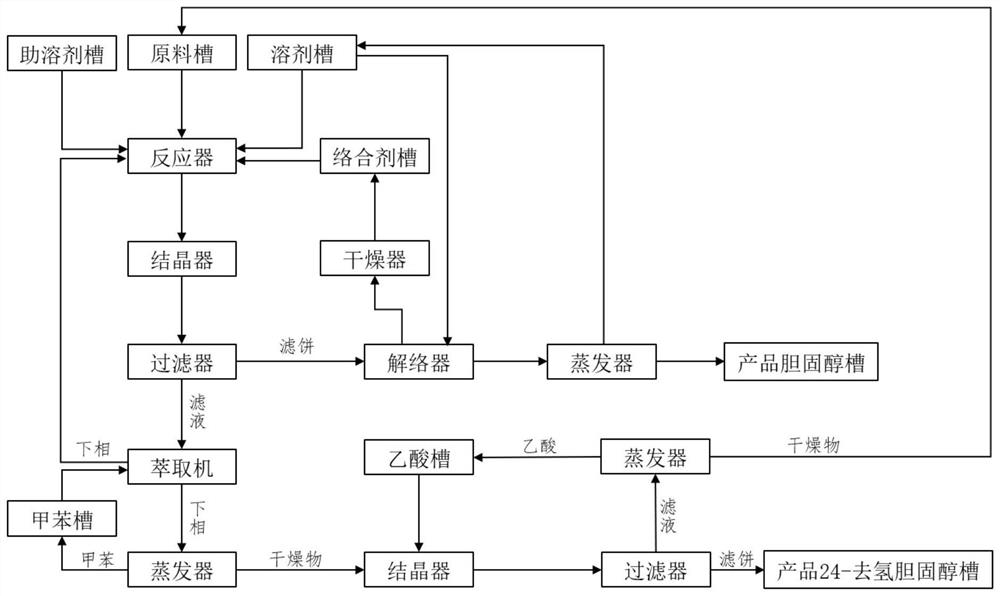
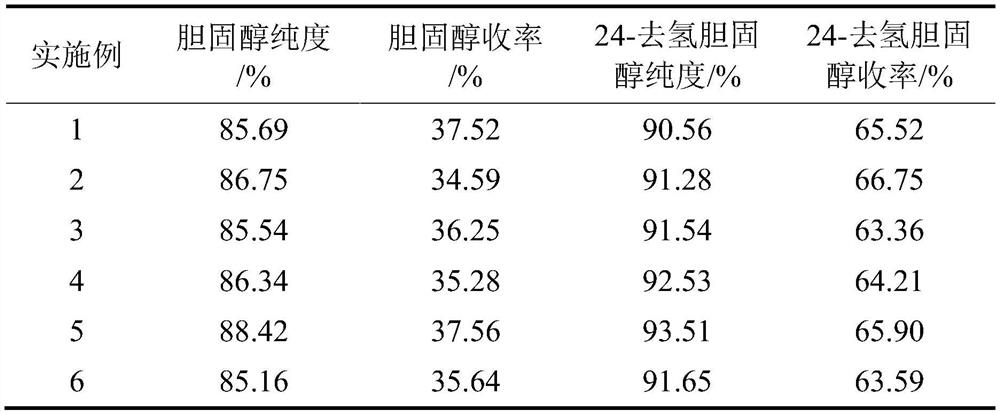
ActiveCN113735931BEfficient separationReduce the number of crystallizationSteroidsAcetic acidCholesterol
The invention discloses a method for separating cholesterol and 24-dehydrocholesterol by complex crystallization. First, soluble cuprous salt is used as a complexing agent to obtain high-purity cholesterol through complex crystallization, and then cholesterol and 24-dehydrocholesterol in the mother liquor are separated by toluene. The dehydrocholesterol was extracted, and the mixture obtained after evaporating to dryness was dissolved in acetic acid for recrystallization to obtain high-purity 24-dehydrocholesterol. Simultaneously, after the mother liquor of acetic acid is evaporated, its dried matter can be used as raw material continuously, thereby improving the whole process yield. The present invention can be separated by using a crystallization reactor commonly used in the field, has low requirements on equipment, and the complexing agent used can greatly improve the separation efficiency, and has the characteristics of simple operation, less solvent consumption, and environmental protection.
Owner:浙江花园营养科技有限公司
A kind of abamectin refining method
ActiveCN110128487BHigh yieldHigh puritySugar derivativesSugar derivatives preparationAbamectinSolvent
The invention discloses a method for purifying abamectin, comprising step 1: adding crude abamectin into a solvent, heating and stirring in an oil bath to dissolve to obtain a crude abamectin solution, the The mass volume ratio of abamectin crude product and described solvent is 1:(4-10), unit g / ml; Step 2: the abamectin crude product solution is carried out the reflux reaction of 1h-2h; Step 3: to step Formamide was added to the crude Abamectin solution that had undergone reflux reaction in the second, cooling and crystallization was carried out, suction filtration was performed to obtain a solid, and the solid was vacuum-dried to obtain a pure Abamectin product. The crystallization times are few, the yield is high, and the oxidation impurities are effectively eliminated.
Owner:江苏海岸药业有限公司
A method for the purification and separation of artemisinin enhanced by chitosan functional membrane
ActiveCN110681182BInhibition of co-precipitationStrengthen the purification and separation processIon-exchange process apparatusOrganic chemistryWaxMedicinal chemistry
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
A kind of sucralose-6-ethyl ester purification method
ActiveCN113366006BHigh purityImprove qualityEsterified saccharide compoundsSugar derivativesAlkaneSucrose
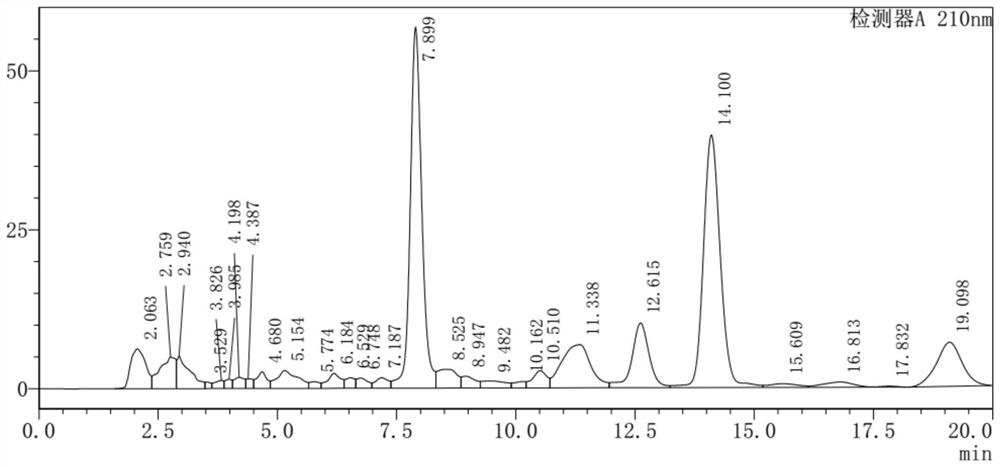
The invention discloses a method for purifying sucralose-6-ethyl ester. The method comprises: a secondary boiling step: taking a boiled and negative-pressure-treated mother liquor of sucralose-6-ethyl ester; ‑Adding a predetermined proportion of water to the ethyl ester mother liquor, fully stirring and heating it for boiling, stirring and boiling for a preset time, and then solid-liquid separation to obtain a secondary boiling mother liquor in which sucralose‑6‑ethyl ester is dissolved; phase-separated extraction step : the secondary boiled mother liquor is left to stand for phase separation, the upper phase after the phase separation is taken, and the alkane extractant is used for extraction at a preset temperature to remove the residual white oil in the upper phase; recrystallization and purification step: extracting the obtained The lower layer effluent obtained is evaporated to obtain a solid, and the solid is purified by recrystallization to obtain sucralose-6-ethyl ester; recovery and circulation step: the upper layer effluent after extraction is separated, and the alkane extractant is recovered and recycled to the phase-separating extraction step use. The method is simple, efficient, and low in cost, can effectively remove trace amounts of white oil, and improve the purity of sucralose-6-ethyl ester.
Owner:ANHUI JINHE IND CO LTD
A kind of method of processing fluoroethylene carbonate solid waste
ActiveCN110775990BEffective protectionHeating evenlyOrganic chemistryAlkali metal chloridesInfraredPotassium hydroxide
The invention discloses a method for treating fluoroethylene carbonate solid waste, comprising the following steps: 1) heating and decomposing fluoroethylene carbonate waste residue at a certain temperature in an infrared device; 2) adding an appropriate amount to the decomposed waste residue The hot water containing a certain amount of potassium hydroxide, stirred and dissolved, concentrated and then crystallized at room temperature for the first time; 3) After the first crystallization was drained, added to clean water, heated to a certain temperature, stirred and dissolved, and concentrated 4) drying the second crystallization at a certain temperature in an infrared device to obtain the by-product potassium chloride. The method for treating fluoroethylene carbonate solid waste provided by the invention adopts far-infrared equipment for preheating, the heating is uniform, the heating speed is fast, the organic matter in the solid slag can be removed, and it is not easy to agglomerate. processing steps, correspondingly reducing processing costs.
Owner:江苏瀚康新材料有限公司
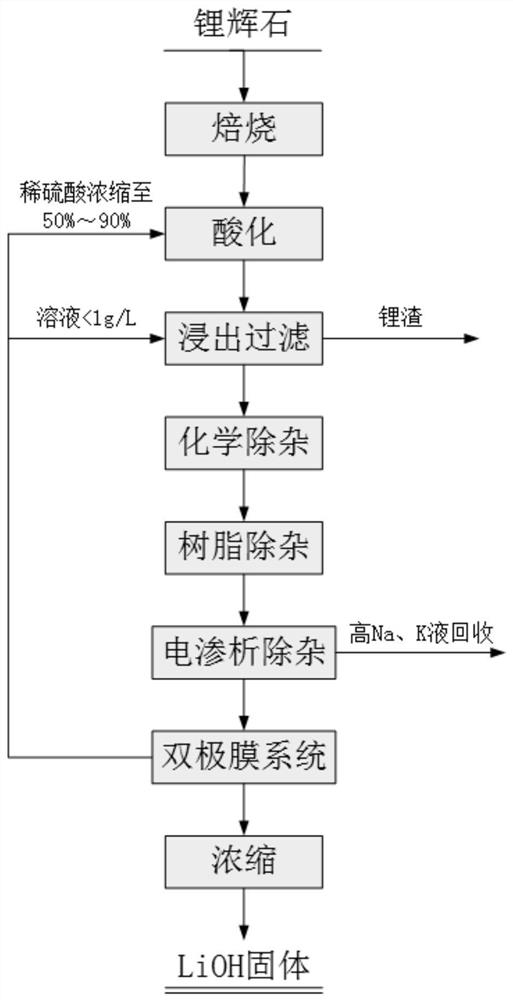
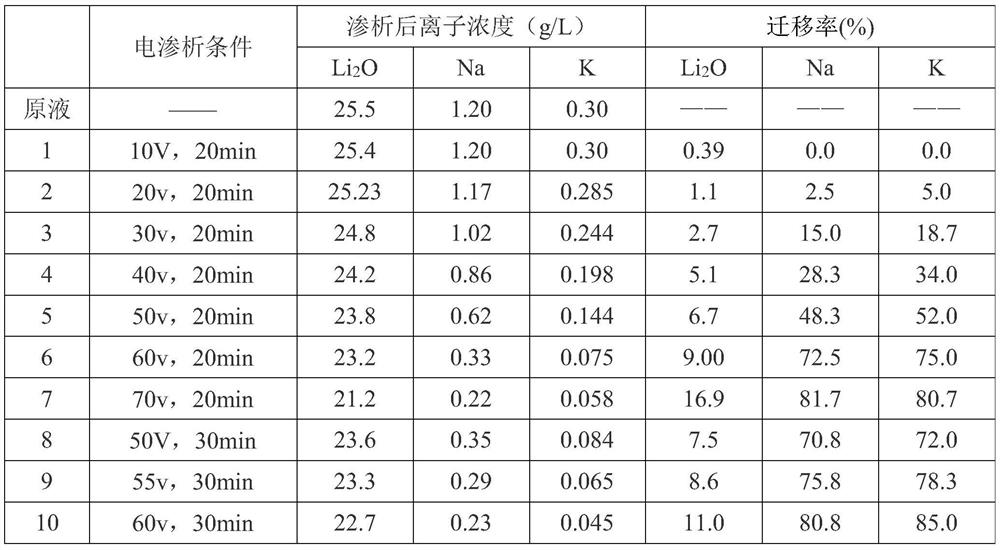
The method for preparing lithium hydroxide from spodumene and the method for removing sodium and potassium
ActiveCN112575339BGood removal effectTo achieve the purpose of removing sodium and potassiumCellsLithium oxideHydrogen Sulfate
The invention relates to a method for preparing lithium hydroxide from spodumene and a method for removing sodium and potassium, and belongs to the technical field of lithium extraction from ores. The technical problem solved by the present invention is to provide a method for preparing lithium hydroxide from spodumene. The method comprises: 1) roasting; 2) acidifying, leaching, and filtering to obtain lithium sulfate mother liquor; 3) removing high-valent metal ions in lithium sulfate mother liquor; 4) removing sodium and potassium by electrodialysis; 5) bipolar membrane electrolysis to obtain hydrogen Lithium oxide solution and dilute sulfuric acid; 6) concentration and crystallization of lithium hydroxide solution to obtain lithium hydroxide product. The method of the present invention can produce battery-grade lithium hydroxide from spodumene, and the method is simple, environmentally friendly and low in cost; at the same time, it does not need to add caustic soda and freeze, and has high economic value. Before bipolar membrane electrolysis, ordinary electrodialysis removes sodium Potassium, so that the purity of the obtained product is greatly improved compared with the traditional method, and the direct crystallization can reach the battery level, which meets the trend and requirements of green and sustainable development.
Owner:天齐锂业(江苏)有限公司
Ganciclovir recovery method
ActiveCN101851239BHigh purityReduce the number of crystallizationOrganic chemistryRecovery methodActivated carbon
The invention relates to a ganciclovir recovery method, which comprises the steps that ganciclovir mother solution is concentrated, alkaline solution is added to adjust pH value to 10.0-13.5, temperature is kept to be constant at 20-60 DEG C for 10-60 minutes, active carbon is added and agitation is conducted for 1-3h, filtration is conducted, organic solution is added in filtrate to precipitate white crystals, filtration is conducted, filter cakes are dissolved in deionized water, hydrochloric acid is added to regulate pH value to 5-6, solid sediment is precipitated, after the solid sedimentis heated and dissolved, the temperature of the solid sediment is slowly decreased by 0-30 DEG C to precipitate crystals, and the crystals are filtered and dried to obtain high-purity ganciclovir products. Compared with the prior art, the invention has the advantages of simple operation, high yield, low cost, no pollution and the like.
Owner:SHANGHAI YIWEI INDAL
Crystallization method of abamectin Bla
The present invention relates to a crystallization method of abamectin B 1a. Said method includes the following steps: using crystallization solvent n-butanol to stir and dissolve primary crude powder of abamectin B 1a at 75-100deg.C to saturation, filtering while the saturated solution is hot to obtain clear hot-saturated solution; slowly cooling said solution to that when the supersaturation degree is 1-3, adding crystal seeds, constant stirring for 20-60min, its stirring speed is 120-300rpm, and cooling to make crystallization, fitering crystal slurry or centrifugally-separating said crystal slurry, washing crystal and drying so as to obtain the invented abamectin B 1a.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Production process of high-purity abamectin fine powder
ActiveCN102617668BReduce consumptionEnhance layeringSugar derivativesSugar derivatives preparationOrganic EsterRoom temperature
The invention relates to a production process of abamectin fine powder. The production process method comprises the steps: extracting fermentation liquid filter cake with sec-butyl acetate for three times; combining organic-ester-phase extracts, adding tetrabutyl ammonium bromide aqueous solution for washing, and filtering, concentrating filtrate, and cooling and crystallizing to obtain once crude product; and dissolving the once crude product with ethanol, filtering, naturally cooling to room temperature, adding water at a volume ratio of ethanol to water being (7 : 1)-(4 : 1) for dilution crystallization, washing and drying to obtain the abamectin fine powder product. By adopting the method, the abamectin crystal product with higher purity and yield can be obtained; and compared with the traditional methods, the method has the advantages that the crystallizing time is shorter, the repeated crystallizing frequency is reduced and the production cost is low, thereby being a low-cost and high-efficiency production process of the high-purity abamectin fine powder.
Owner:DAQING JEFENE BIO CHEM
A kind of preparation method of Abamectin b2a
ActiveCN104650167BHazard reductionReduce pollutionSugar derivativesSugar derivatives preparationAvermectin B1aFiltration
The invention relates to a preparation method of high-purity abamectin B2a, which comprises the steps of concentrating the abamectin B1a crystallization mother liquor under vacuum conditions to no fraction to obtain a thick ointment; adding an extractant for extraction to obtain an extract , and then use saturated brine to wash the extract for 2 to 3 times, remove the water phase to obtain n-butyl acetate solution; cool the obtained n-butyl acetate solution to crystallize, grow crystals, and suction filter to obtain crude crystals of abamectin B2a ; Abamectin B2a coarse crystal recrystallization suction filtration, drying, to obtain high-purity avermectin B2a fine powder. The present invention uses the environment-friendly non-toxic solvent n-butyl acetate to replace the toxic solvent aromatic hydrocarbon to produce abamectin B2a high-quality goods. During the production operation, it causes little harm to the health of on-site employees, and the pollution to the environment is also relatively small. In addition, n-butyl acetate is adopted as B2a as crystallization solvent, and the purity of the obtained abamectin B2a fine powder is above 95%.
Owner:QILU PHARMA INNER MONGOLIA
Preparation method of griseofulvin
The invention discloses a preparation method of griseofulvin, which is composed of the following steps: A, heating the griseofulvin fermented liquid, adding calcium oxide to adjust the pH, separating the solid and liquid from the plate and frame, adding calcium oxide to the filter cake to granulate, Extract with acetone to obtain griseofulvin acetone extract; B. Griseofulvin acetone extract is decolorized by activated carbon column to obtain griseofulvin acetone decolorization liquid, and the decolorization liquid enters the concentration tank, concentrates and crystallizes to obtain griseofulvin wet crystals C, griseofulvin wet crystals are washed once with dichloromethane and ethanol mixed solution under stirring, and then washed once with the medicinal ethanol of 95% with concentration under stirring, dried in a vacuum drier, ultrafinely pulverized, Get griseofulvin products. Compared with the traditional preparation method, the present invention reduces the number of crystallization times in the process of preparing griseofulvin in the prior art, effectively avoids the yield loss caused by the recrystallization of griseofulvin, and can increase the total yield of griseofulvin Increased by 7 to 10%, greatly reducing production costs.
Owner:内蒙古格林特制药有限责任公司
A method for preparing high-purity stigmasterol from phytosterol oleate
ActiveCN107722099BTake advantage ofReduce the number of crystallizationSteroidsPlant sterolFractionation
The invention discloses a method for preparing high-purity stigmasterol from phytosterol oleate. The method comprises the following steps of taking phytosterol oleate as a raw material, and performingsubzero fractionation, saponification, solid-liquid separation and two times of crystallization and purification to obtain a stigmasterol product with purity equal to 95% or more. Compared with the prior art, in the method, the phytosterol oleate is taken as the raw material to prepare the high-purity stigmasterol, the method has fewer crystal number of times and is simple in operation steps, thesolvent used for crystallization and purification can be recycled, making full use of resources is realized, and the production cost is lowered.
Owner:XIAN HEALTHFUL BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com