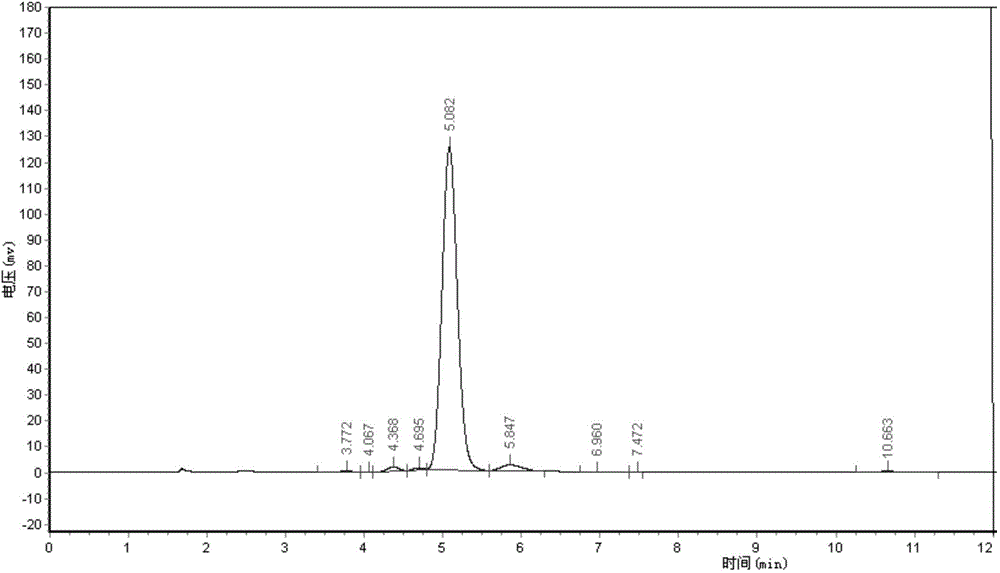
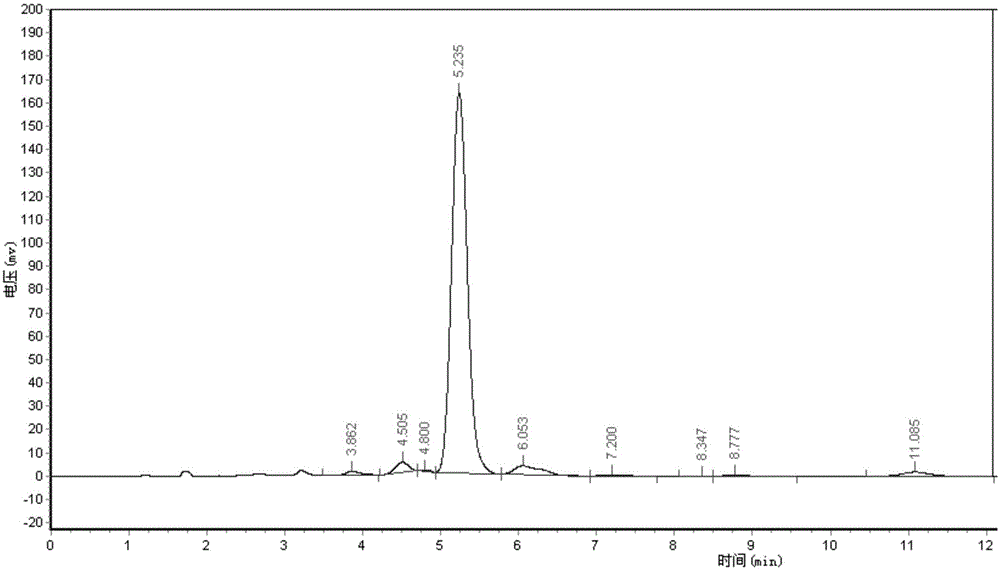
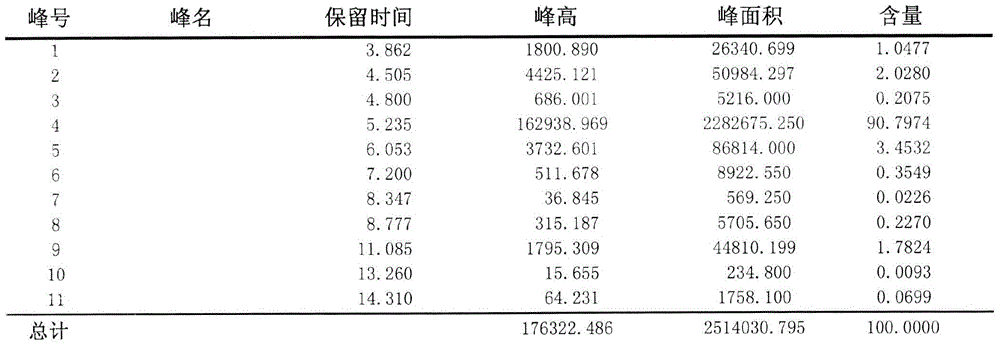
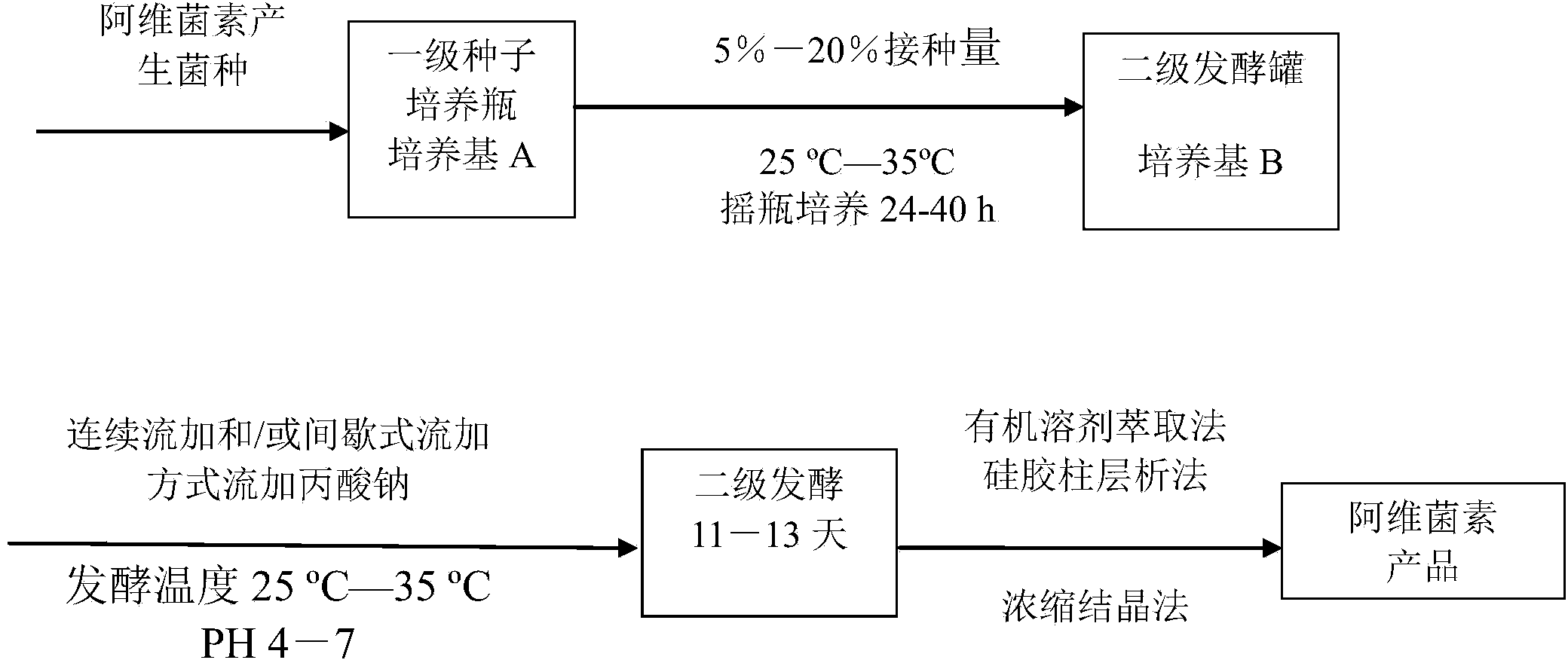
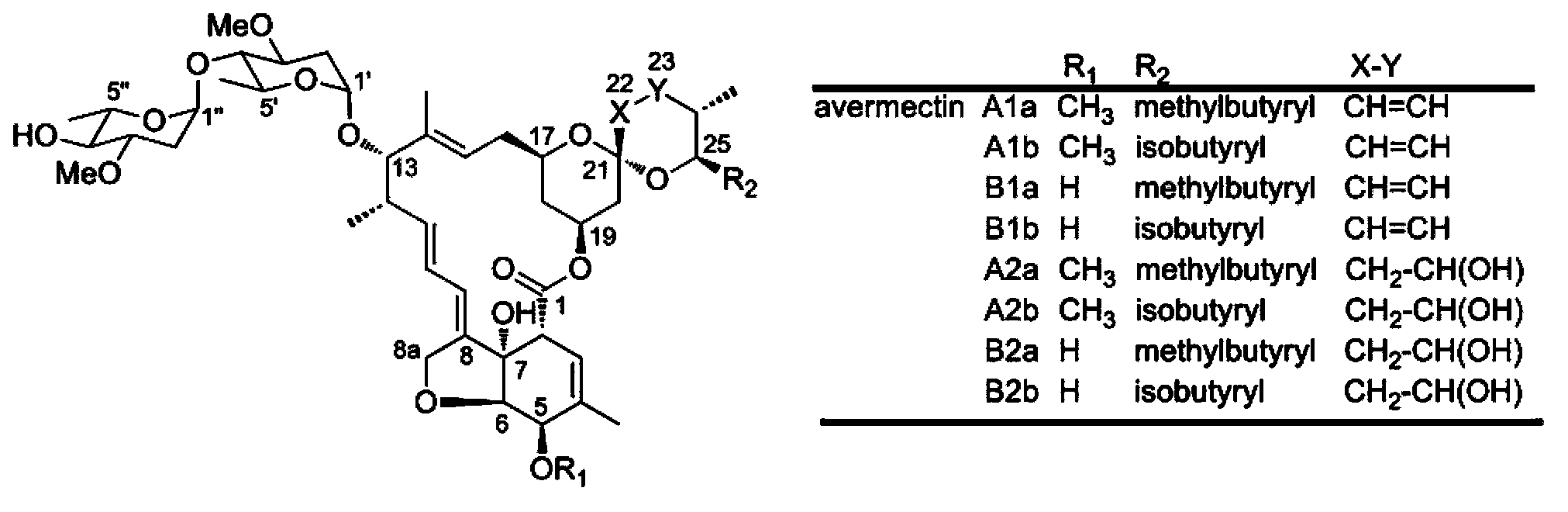
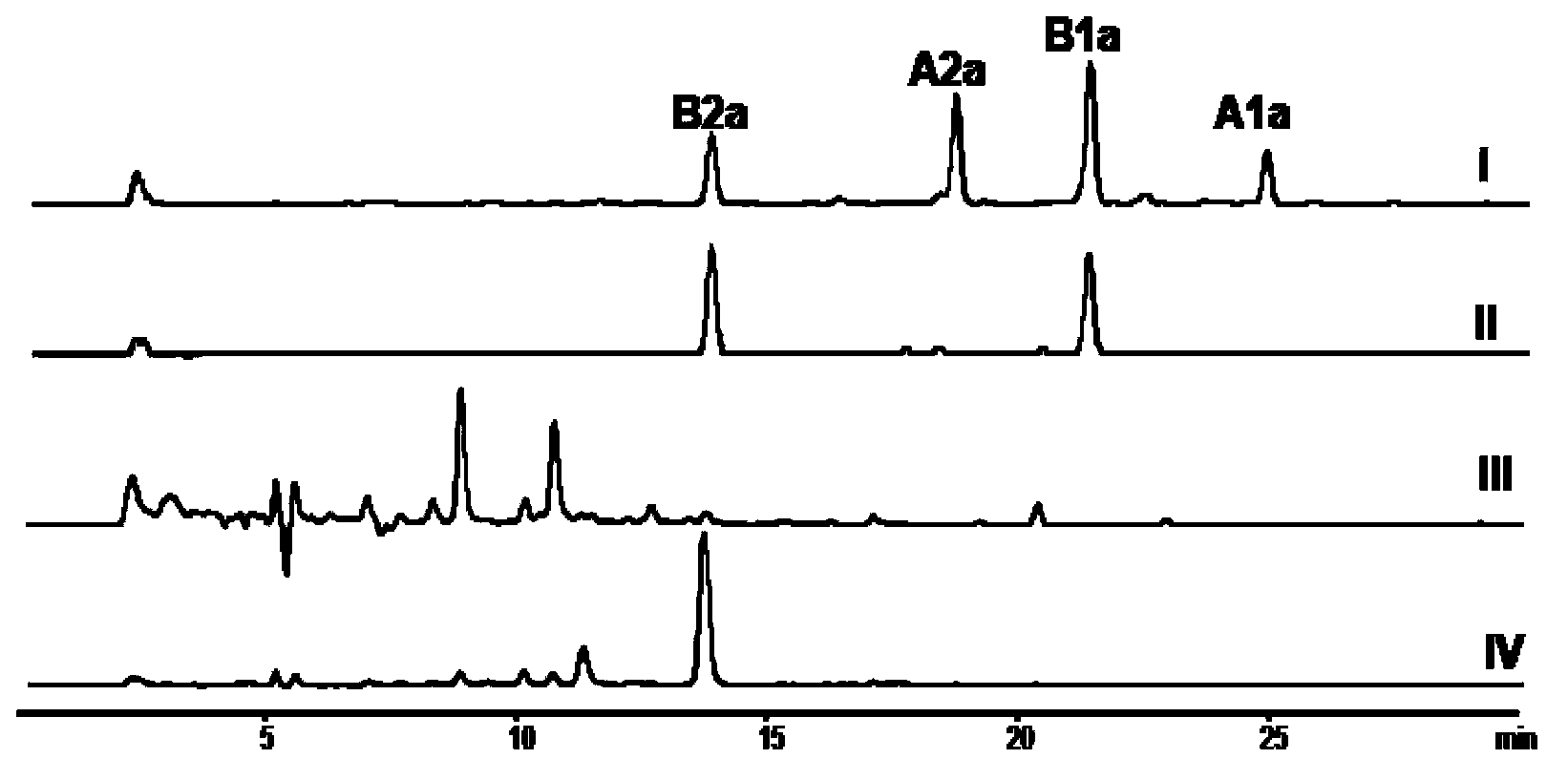
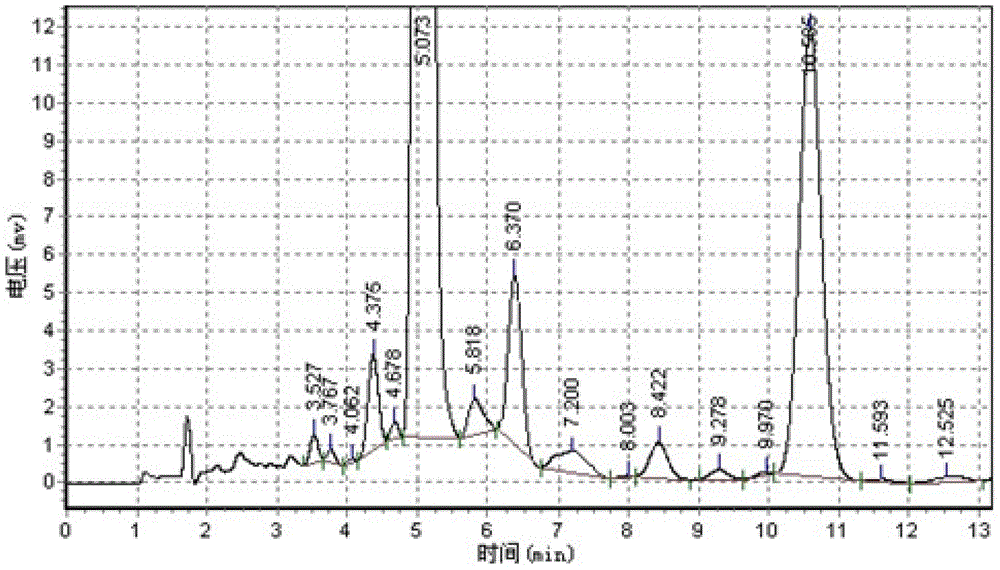
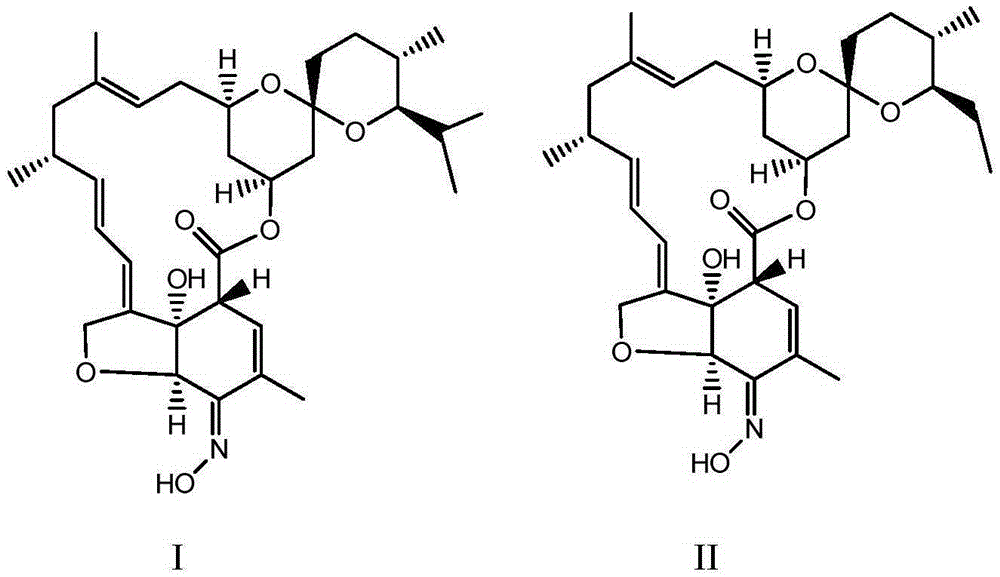
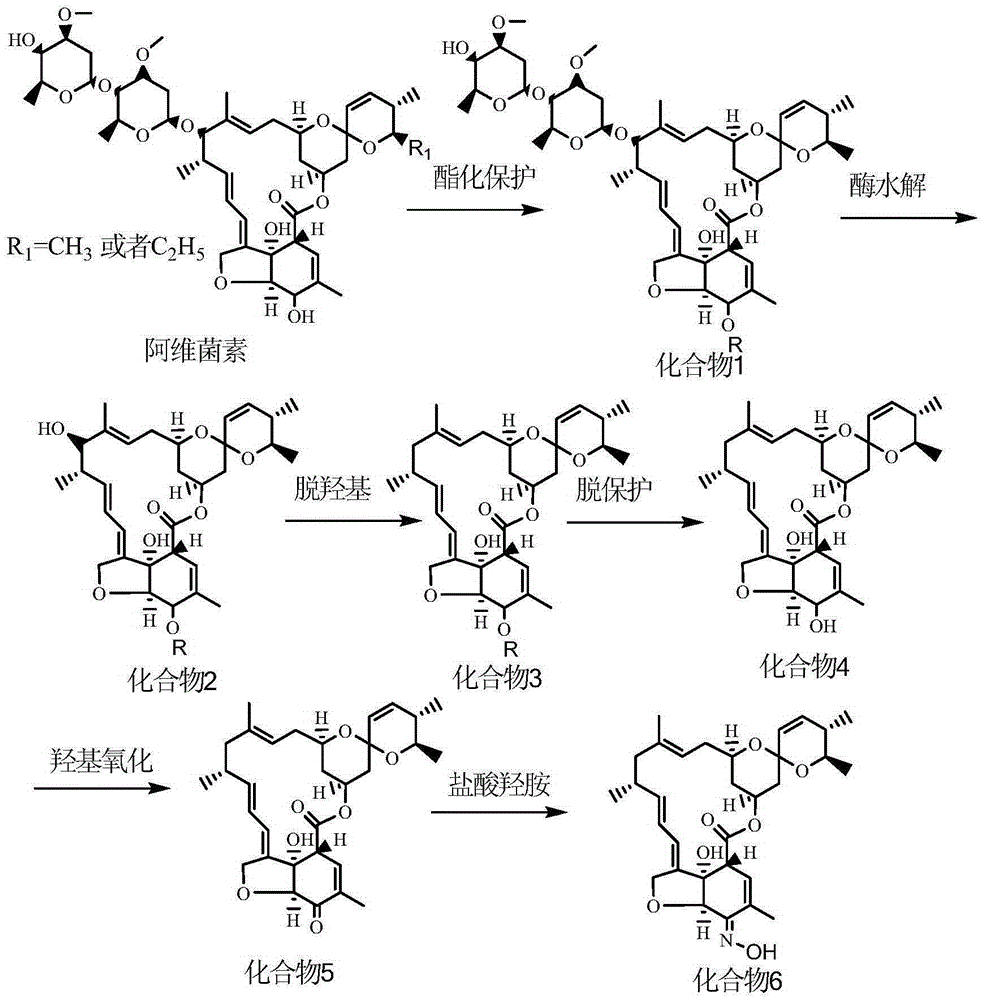
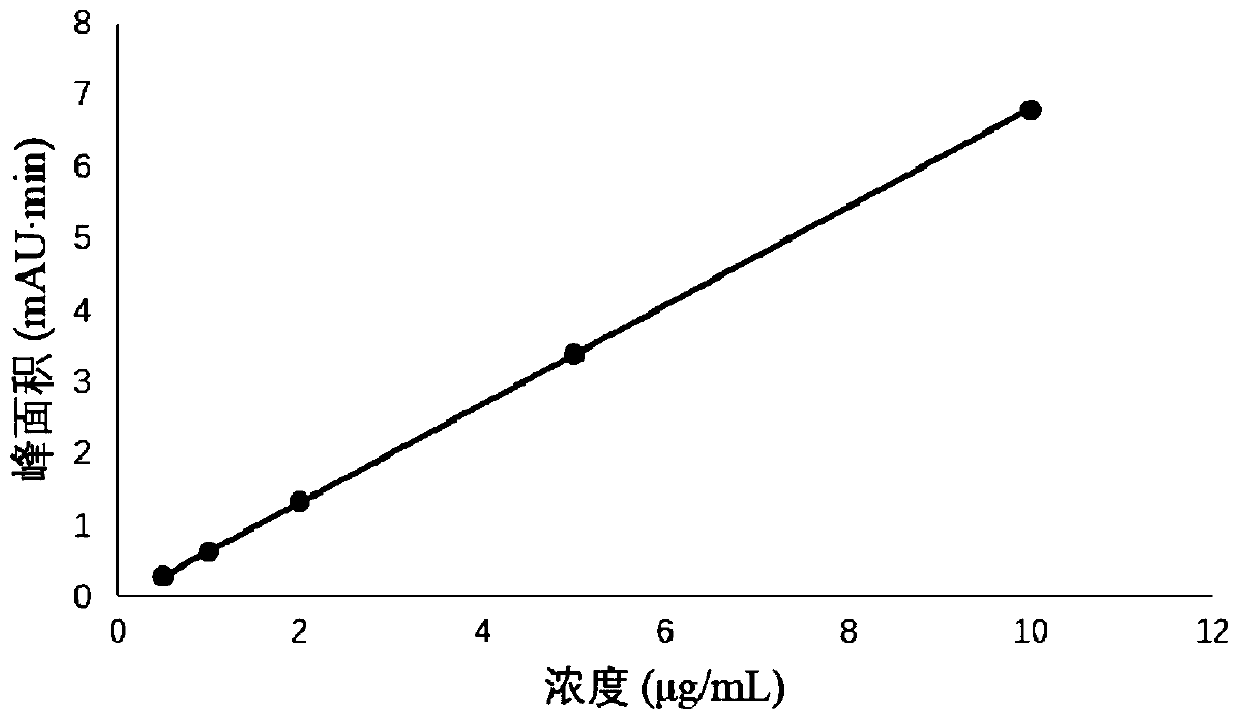
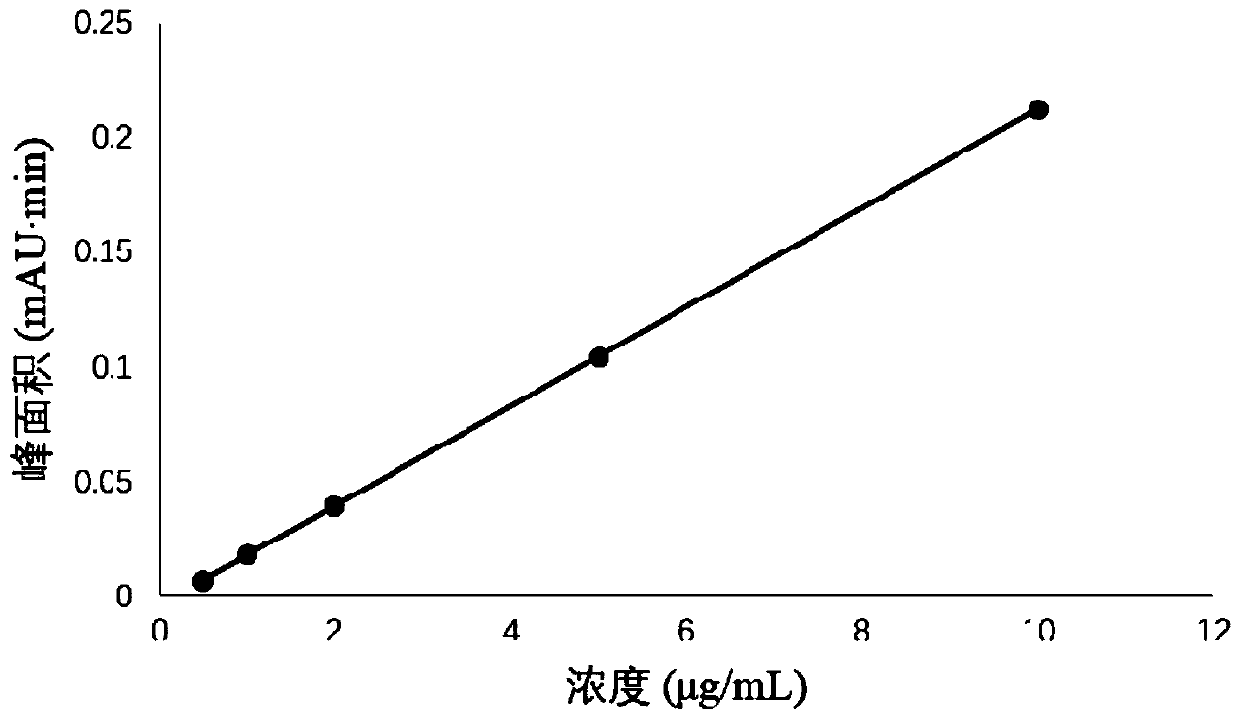
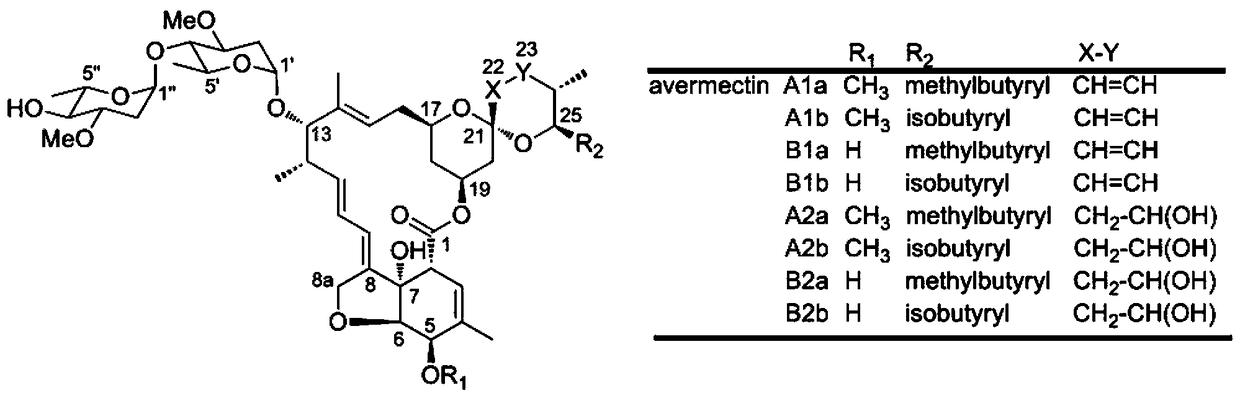
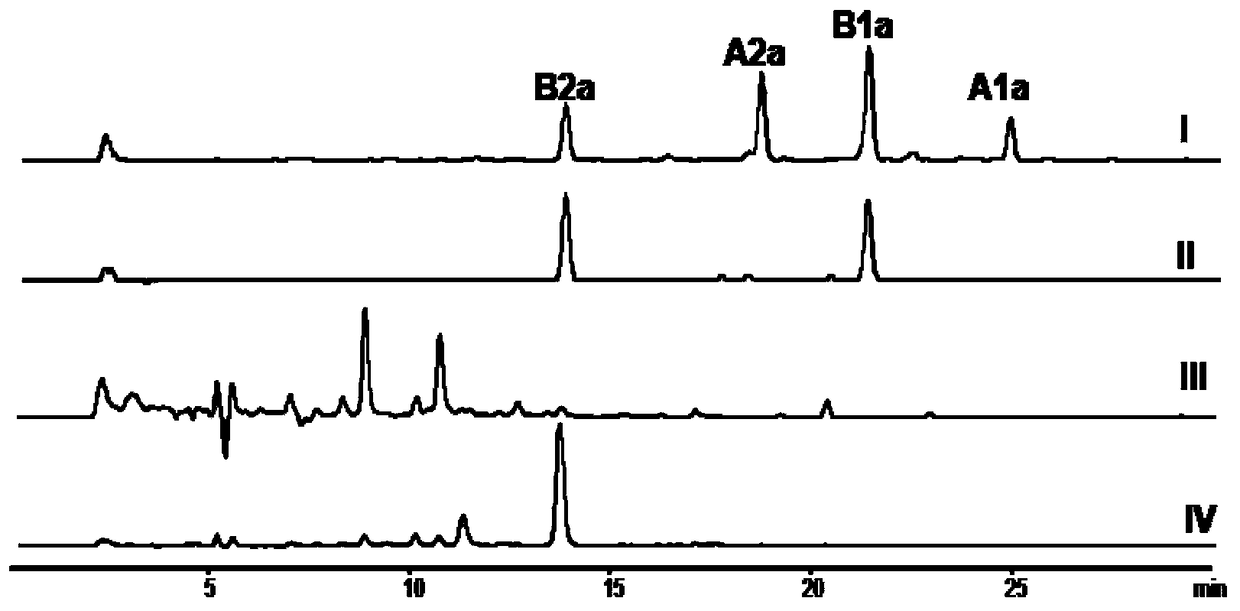
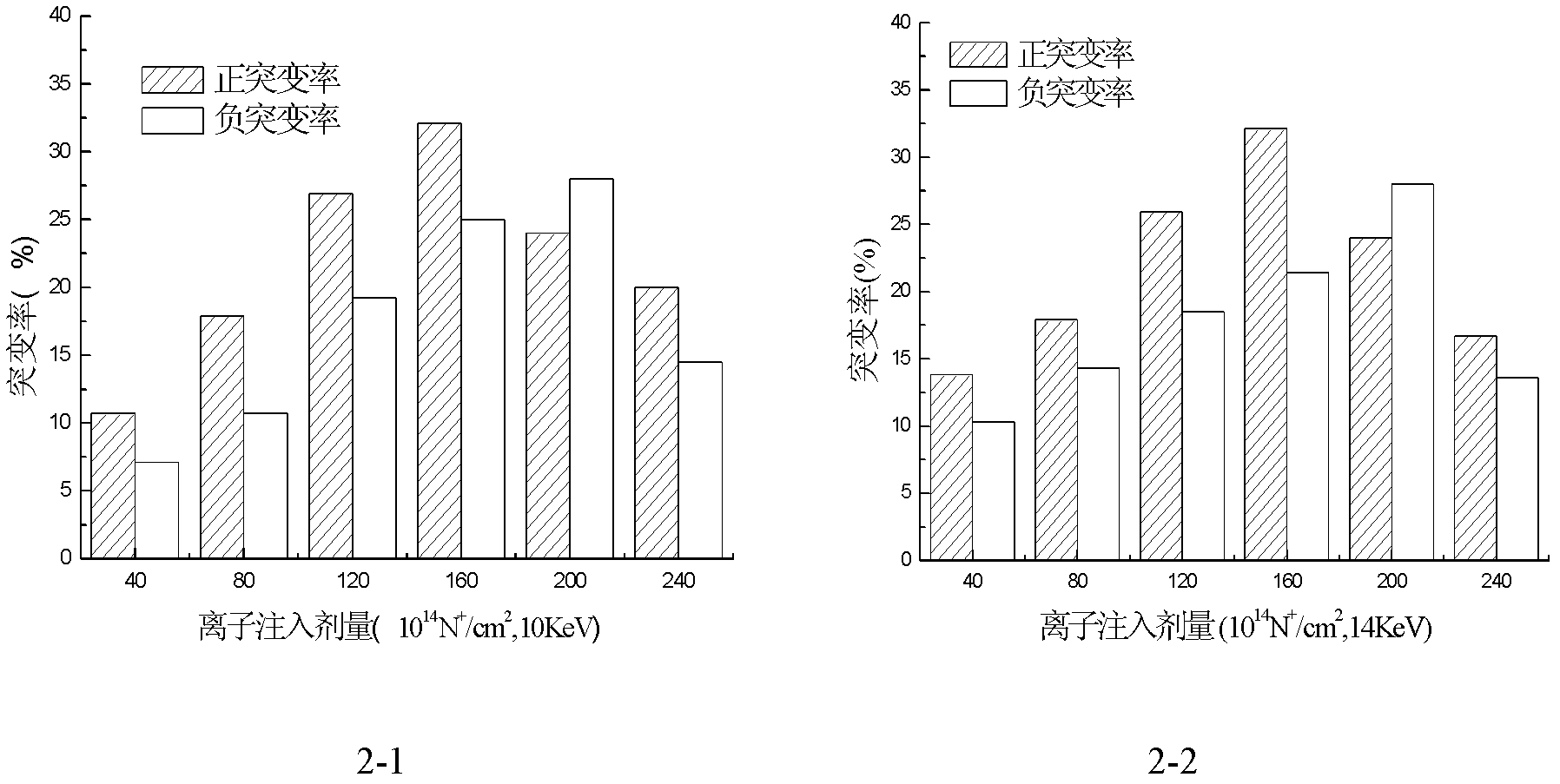Patents
Literature
41 results about "Avermectin B1a" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of Avermectin B2a fine powder
ActiveCN103333214AEnhance layeringGood cleaning effectSugar derivativesSugar derivatives preparationDissolutionSolvent
The invention relates to a preparation method of an Abamectin B2a fine powder. The method comprises the following steps: A, concentrating an Abamectin B1a crystallization mother liquor into a paste state; B, dissolving the obtained paste with sec-butyl acetate, and then adding a tetrabutyl ammonium bromide aqueous solution for washing and layering; C, concentrating the sec-butyl acetate solution subjected to washing and layering, then adding a crystallization solvent for dissolution, naturally cooling to room temperature, crystallizing, and filtering to obtain an Abamectin B2a fine powder crude product; and D, recrystallizing the Abamectin B2a fine powder crude product to obtain the Abamectin B2a fine powder. According to the invention, waste Abamectin crystallization mother liquor is recovered and utilized to extract effective substances; tetrabutyl ammonium bromide aqueous solution is used for washing, which has better impurity removal effect than traditional water washing or saturated brine washing, and makes the solution layer more easily; aromatic hydrocarbon is used as the crystallization solvent; and the content of obtained B2a fine powder is higher than 93%.
Owner:DAQING JEFENE BIO CHEM
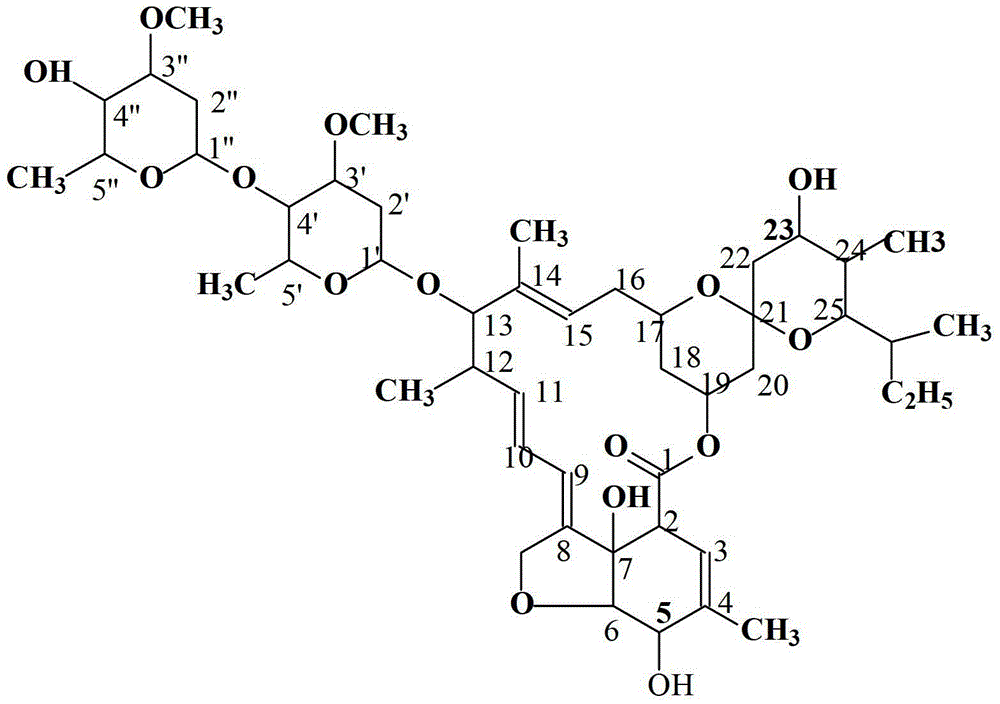
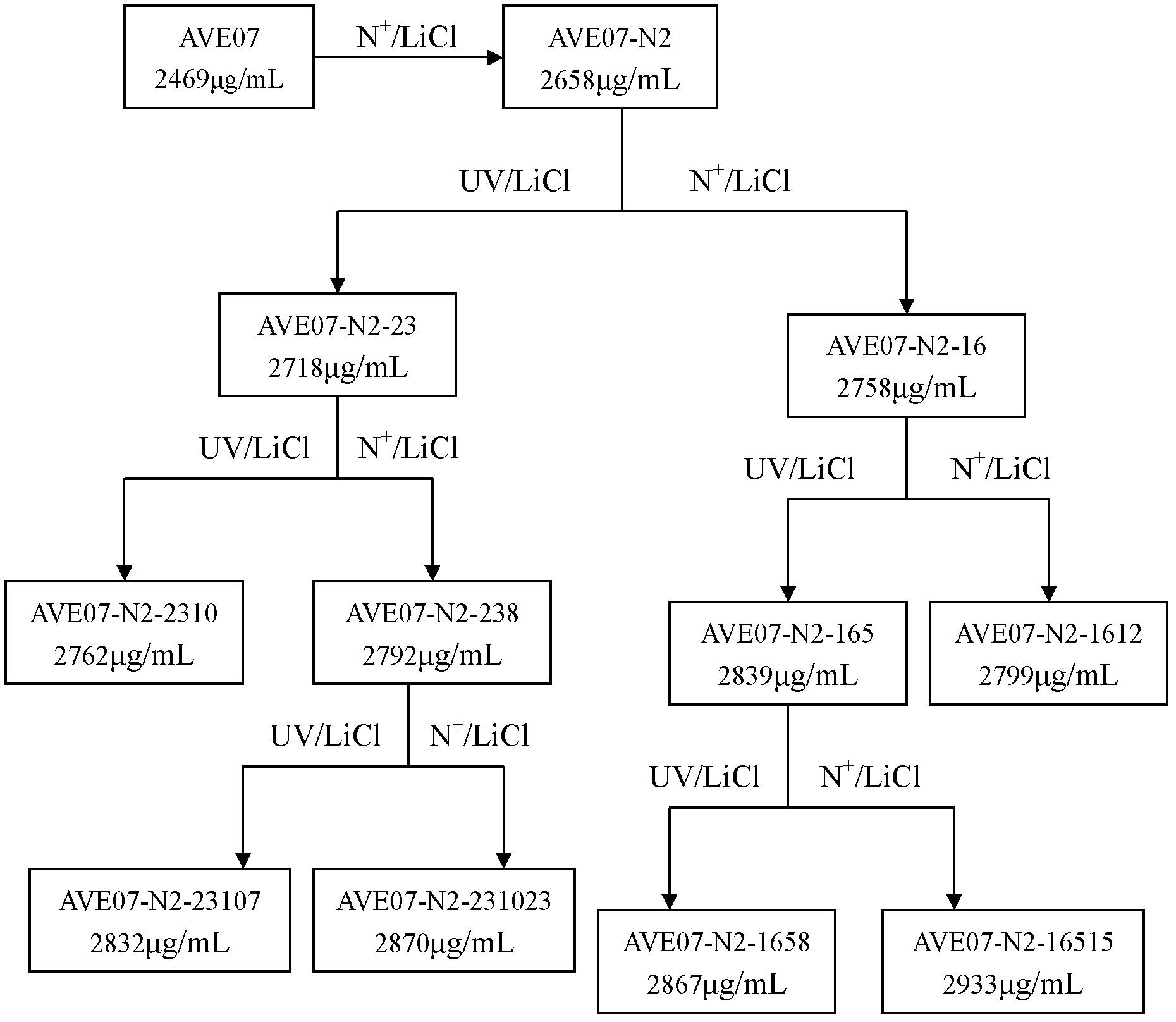
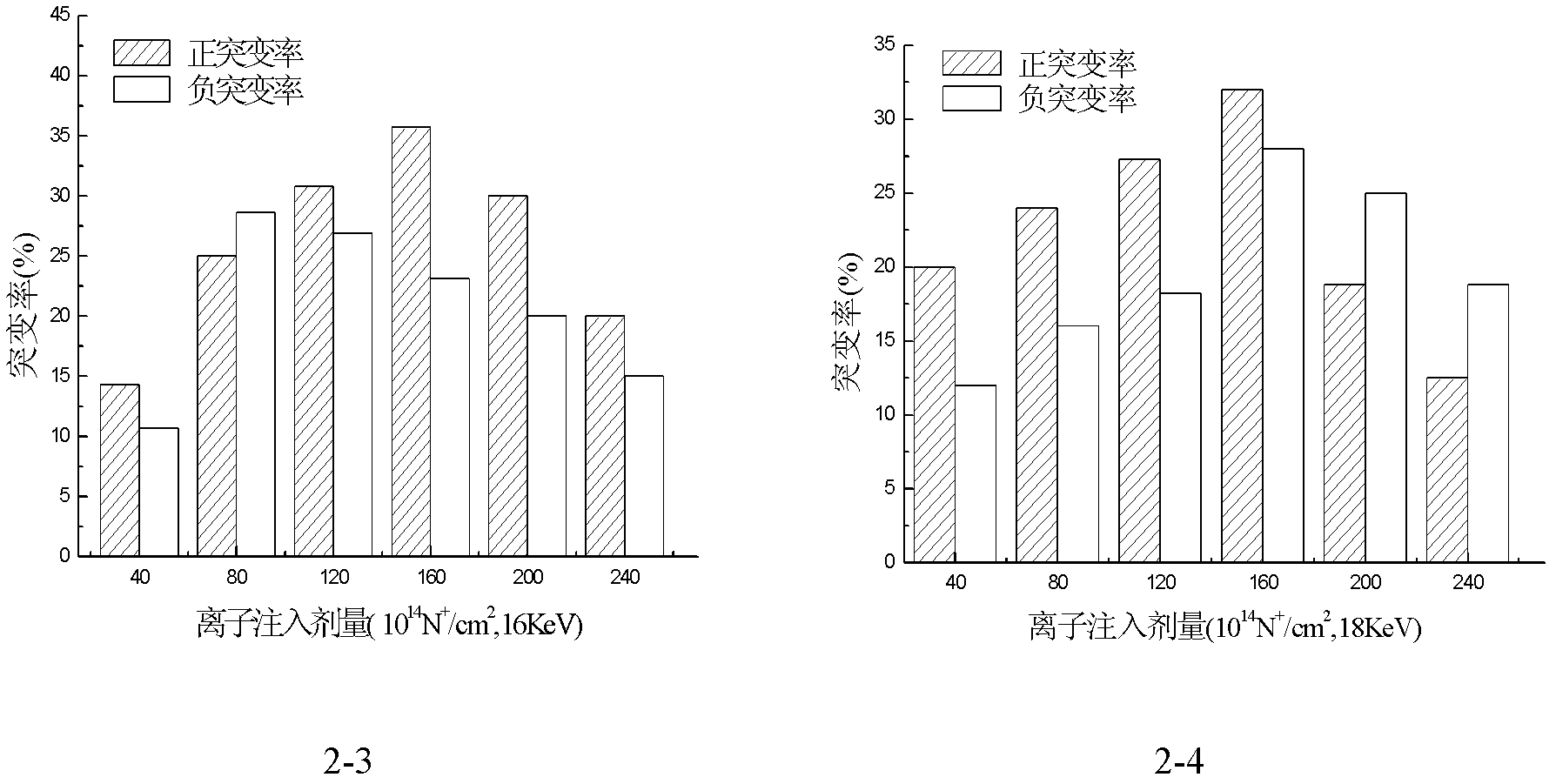
Avermectin B1a high-yielding strain and application thereof
InactiveCN102634471AHigh compositionReduce other componentsBacteriaMicroorganism based processesLithium chlorideUltraviolet
The invention discloses an avermectin B1a high-yielding strain, which is classified and named streptomyces avermitilis AVE 07-N2-16515, and is preserved in a China type culture preservation centre (CCTCC) with the preservation number of CCTCC NO: M2012094. The avermectin B1a high-yielding strain disclosed by the invention is obtained by combining low-energy nitrogen ion implantation-lithium chloride (N<+>-LiCl) compound mutation with ultraviolet ray-lithium chloride (UV-LiCl) compound mutation, primarily screening, and performing shake-flask fermentation secondary screening, and is used as a starting strain for next mutation, so that the target strain AVE07-N2-16515 is finally screened. The obtained strain can greatly increase the avermectin B1a component and reduce other components in fermentation products, and is good in hereditary stability. The strain is fermented in a 5L fermentation tank to produce avermectin B1a by utilizing glucose as a quick-acting carbon source and cornstarch as a delayed-action carbon source, the titer can achieve 3048 mu g / m, which is improved by 23.4% as compared with the original starting strain AVE07; and the strain can be applied on industrial production, greatly improves a fermenting unit, and has great economic application value.
Owner:NANJING UNIV OF TECH
Crystallization method of abamectin Bla
The present invention relates to a crystallization method of abamectin B 1a. Said method includes the following steps: using crystallization solvent n-butanol to stir and dissolve primary crude powder of abamectin B 1a at 75-100deg.C to saturation, filtering while the saturated solution is hot to obtain clear hot-saturated solution; slowly cooling said solution to that when the supersaturation degree is 1-3, adding crystal seeds, constant stirring for 20-60min, its stirring speed is 120-300rpm, and cooling to make crystallization, fitering crystal slurry or centrifugally-separating said crystal slurry, washing crystal and drying so as to obtain the invented abamectin B 1a.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method of high-purity abamectin B2a
ActiveCN104650167AHazard reductionReduce pollutionSugar derivativesSugar derivatives preparationAvermectin B1aFiltration
The invention relates to a preparation method of high-purity abamectin B2a, which comprises the following steps: concentrating an abamectin B1a crystal mother solution to no fraction under vacuum conditions, thereby obtaining an ointment thick material; adding an extractant, extracting to obtain an extract, washing the extract with a saturated saline solution 2-3 times, and removing the water phase to obtain an n-butyl acetate solution; cooling the obtained n-butyl acetate solution to crystallize, growing the grain, and carrying out vacuum filtration to obtain an abamectin B2a coarse crystal; and recrystallizing the abamectin B2a coarse crystal, carrying out vacuum filtration, and drying to obtain the high-purity abamectin B2a refined powder. The environment-friendly nontoxic solvent n-butyl acetate is used instead of the toxic solvent aromatic hydrocarbon to produce the abamectin B2a refined product, so the harm to the in-field personnel body is low in the production operation process, and the environmental pollution is small. By using the n-butyl acetate as the crystallizing solvent of the B2a, the purity of the abamectin B2a refined powder is higher than 95%.
Owner:QILU PHARMA INNER MONGOLIA
Effective method for preparing avermectin
InactiveCN103882080AStrong ability to produce abamectinIncrease productionMicroorganism based processesFermentationBiotechnologyMicroorganism
The invention belongs to the technical field of microorganisms, and particularly relates to a method for preparing avermectin (Avermectins). The method comprises the following steps: cultivating a streptomyces avermitilis strain capable of generating avermectin and genetic improved bacteria thereof in a first stage of fluid nutrient medium, and then inoculating into a second stage of fluid nutrient medium as a seed solution; fermenting and cultivating after inoculating the seed solution into the second stage of fluid nutrient medium, and feeding a sodium propionate precursor to carry out feeding cultivation; collecting the avermectin B1a from fermentation broth after fermentation is finished. The effective method has the characteristics of being high in production efficiency and the like.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
Microcapsule made from avoparcin and its interface polymeric production
An abamectin microsoftgel for preventing and eliminating the pests of agricultural crops is prepared from the abamectin as core and wall material by interface polymerization method. Its advantages are low cost, slow release and low poison.
Owner:GUANGXI RES INST OF CHEM IND CO LTD
Avermectin producing bacteria and preparation method and application thereof
The invention relates to avermectin producing bacteria and a preparation method and application thereof. Specifically, aveD gene in wild type avermectin producing bacteria is inactivated to obtain a strain getting rid of an A component and only producing a B component; by anaplerosis of a homologous gene of the aveC gene (preferably meiC gene in a Meilingmycin biosynthetic gene cluster) on the basis of simultaneous inactivation the aveD gene and aveC gene, 95wt% above of the obtained mutant fermentation product is avermectin B2a. The product avermectin B2a can be used as a precursor for being directly used for large-scale synthesis of high purity avermectin B1a and ivermectin.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Method preparing abamectin Bla fine powder by secondary crystallization in abamectin Bla
ActiveCN104876991AReduce solubilityDissolution achievedSugar derivativesSugar derivatives preparationCrystallographyAvermectin B1a
The invention relates to a method preparing abamectin Bla fine powder by secondary crystallization in abamectin Bla. The method comprises the steps of condensing an abamectin Bla crystallization mother liquid until no fraction is generated in a vacuum condition, so as to obtain a thick ointment slurry; adopting sec-Butyl acetate as an extraction agent, growing the grain to obtain a plurality of abamectin Blas when the temperature is risen to 90-95 DEG C and the stirring speed is 10-20 r / min, therefore the yield of the abamectin Bla in the abamectin Bla crystallization mother liquid can be improved.
Owner:QILU PHARMA INNER MONGOLIA
Milbemycin oxime compound and preparation method thereof
InactiveCN103896961AHigh yieldSimple and fast operationOrganic chemistryNMR - Nuclear magnetic resonanceAvermectin B1a
The invention discloses a milbemycin oxime compound and a preparation method thereof. The compound as shown in a general formula (I) in the specification is prepared by the steps of carrying out hydrolysis reaction on avermectin B1a to remove C13-position glycosyl, oxidizing both C5-position hydroxyl and C13-position hydroxyl into keto through oxidation reaction, and finally converting the two kinds of keto into oximido, wherein the structure of the compound is confirmed by virtue of the mass spectrum and nuclear magnetic resonance analysis testing technology. According to the milbemycin oxime compound and the preparation method, the milbemycin oxime compound is prepared by starting materials which are cheap and easily obtained; the preparation method has the characteristics of mild reaction condition, simpleness in operation process, low cost and higher yield.
Owner:WUHAN UNIV
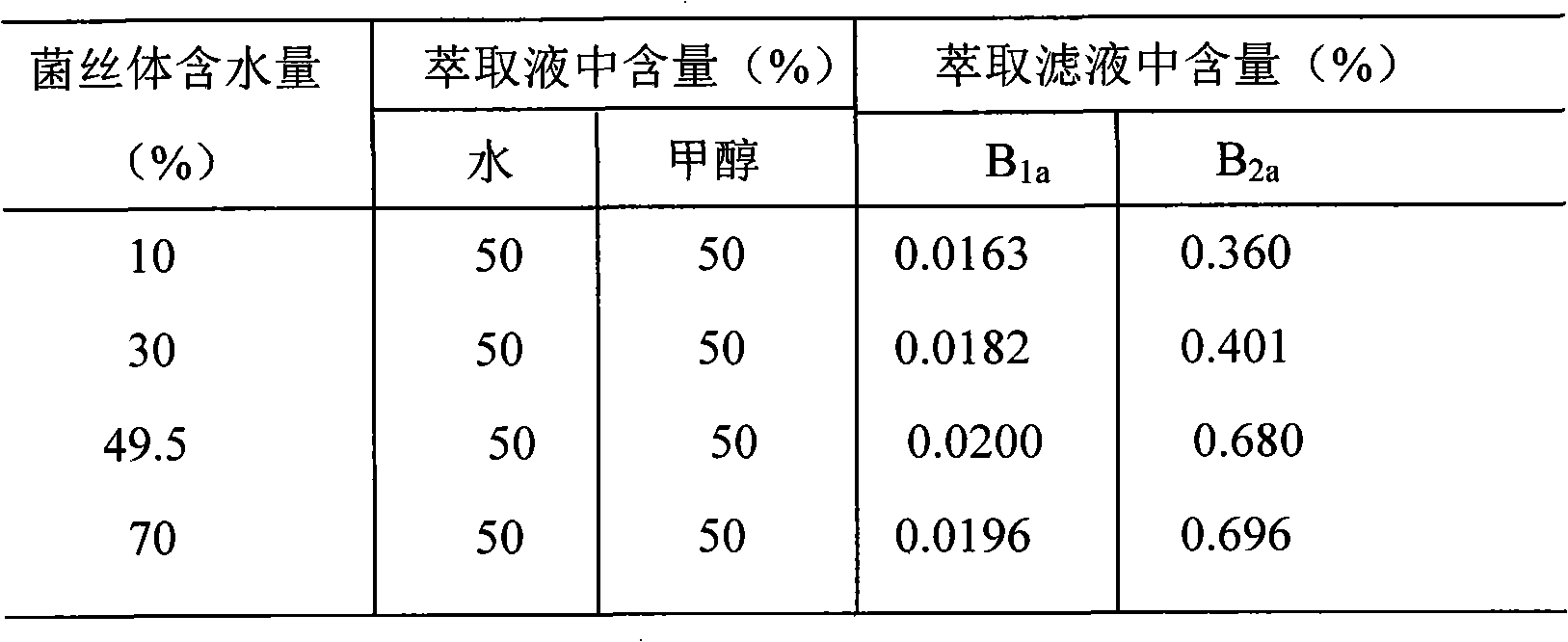
Method for step by step extracting avermectin B1a and B2a from mycelium
The invention discloses a method for selectively extracting avermectin B1a and B2a from streptomyces avermitilis mycelium, and the method is prominently characterized in that the avermectin B1a and B2a are extracted from the mycelium in two steps, first water / methanol or water / ethanol cosolvent is used for selective extraction of the B2a from the mycelium, and then an organic dissolvent such as methanol or ethanol and the like is used for extraction of the B1a from the mycelium. The method can effectively separate the B1a and the B2a, facilitates subsequent separation and purification of the B1a and the B2a, and can enhance the crystallization yield of the B1a and the B2a.
Owner:王玉万 +2
Method for extracting residual avermectin B1a from primary crystallization mother liquor of avermectin B1a
ActiveCN105418708ASolve eager technical problemsIncrease contentSugar derivativesSugar derivatives preparationKetoneSolvent
The invention discloses a method for extracting residual avermectin B1a from primary crystallization mother liquor of avermectin B1a. The method comprises the steps that low-grade ketone is added to avermectin factice, heating and stirring are conducted to make the low-grade ketone dissolved, cooling and centrifugation are conducted, obtained supernatant is recycled and concentrated, and second factice is obtained; crystallization solvent is added to the second factice which is dissolved through heating, cooling is conducted to enable crystals to grow, the crystals are separated, and coarse powder is obtained; methanol is added to the coarse powder, then activated carbon is added for decoloring treatment, filtrate is recrystallized twice, and avermectin B1a fine powder with the content of the avermectin B1a larger than or equal to 95% is obtained. By means of the method for extracting the residual avermectin B1a from the primary crystallization mother liquor of the avermectin B1a, the B1a component in the primary crystallization mother liquor can be effectively recycled, 86% of the B1a component in the mother liquor can be recycled through the primary crystallization, and the content of the B1a component in the obtained coarse powder reaches up to 65%. In addition, the method has the advantages of being simple in process, low in cost and suitable for the needs of industrial production and meets the industrial requirements, and meanwhile the realistic problem that the factice is banned from using nationally is solved.
Owner:HEBEI XINGBAI AGRI SCI & TECH CO LTD
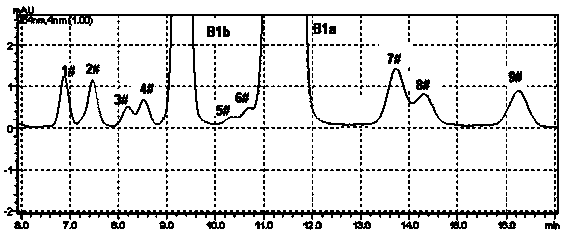
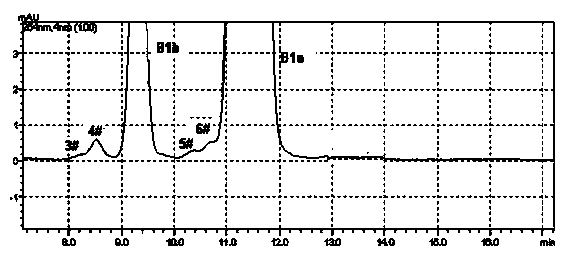
Method for preparing high-purity Abamectin
ActiveCN103641873AEfficient removalHigh puritySugar derivativesSugar derivatives preparationAvermectin B1aSolvent
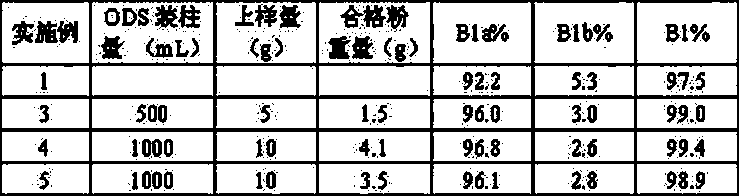
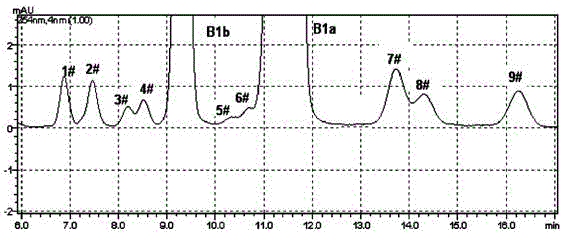
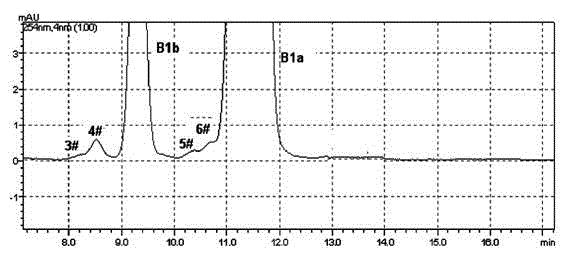
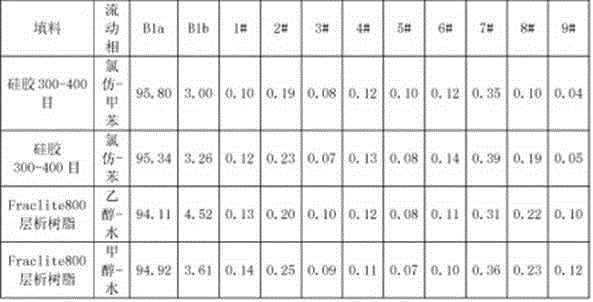
The invention discloses a method for preparing high-purity Abamectin. The method comprises the following steps: dissolving Abamectin coarse powder in acetone, loading a sample to a chromatographic column filled with octadecyl silane bonded silica gel inversed phase chromatography fillers, gradient eluting the sample by using mixed liquid of a polar solvent and water as eluent, and carrying out stepwise collection, liquid phase detection, mixing, concentration and drying to obtain the target product, the purity of Abamectin B1 in the target product is 98.5-99.5%, and the purity of Abamectin B1a is not larger than 97% while not smaller than 95%. The component proportions of Abamectin B1a and Abamectin B1b in the final product of the invention are controllable, B1b is not larger than 5.0%, the other impurities 1# to 9# are not larger than 0.1%, the process is simple, the yield is stable, the quality is controllable and safer technical support is provided for production of pharmaceutical grade raw materials.
Owner:NORTH CHINA PHARMA GROUP AINO
Method used for increasing industrial avermectin B<1a> yield via optimization of fermentation medium
ActiveCN106609288AIncrease productionMicroorganism based processesFermentationAvermectin B1aChloride
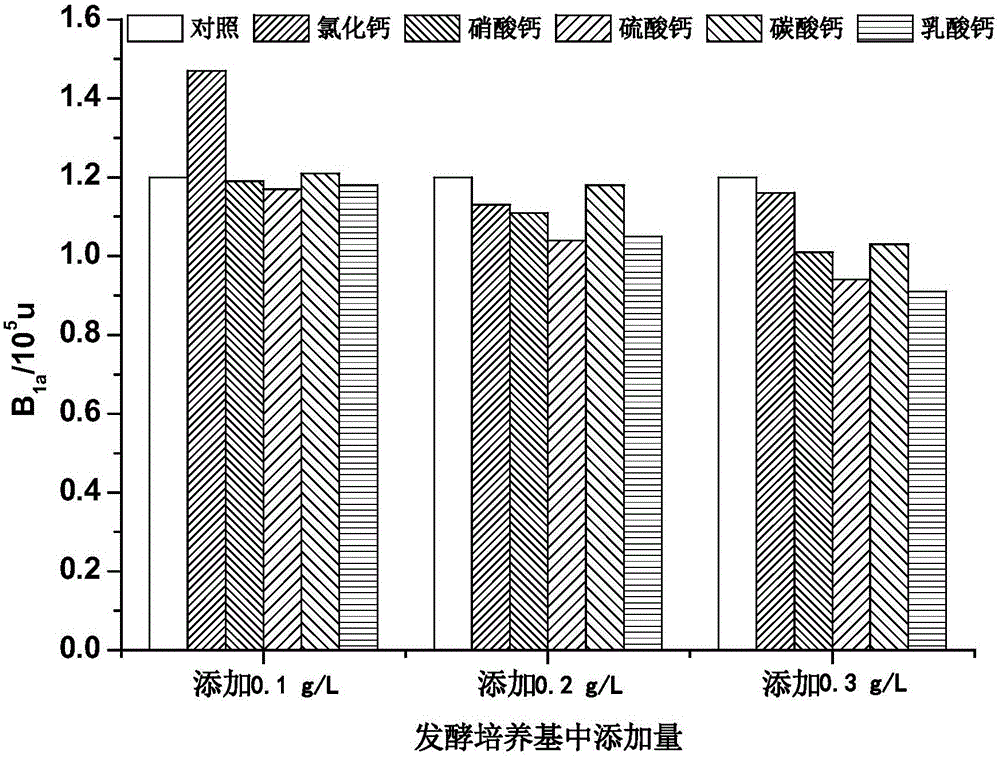
The invention relates to a method used for increasing industrial avermectin B<1a> yield via optimization of fermentation medium. It is found via large scale of screening that adding of an appropriate concentration of calcium chloride into streptomyces avermitilis fermentation medium is capable of increasing B<1a> yield obviously. The invention also provides a medium used for the method.
Owner:上海国佳生化工程技术研究中心有限公司 +1
A kind of preparation method of Abamectin
ActiveCN103641873BEfficient removalHigh puritySugar derivativesSugar derivatives preparationAvermectin B1aSolvent
Owner:NORTH CHINA PHARMA GROUP AINO
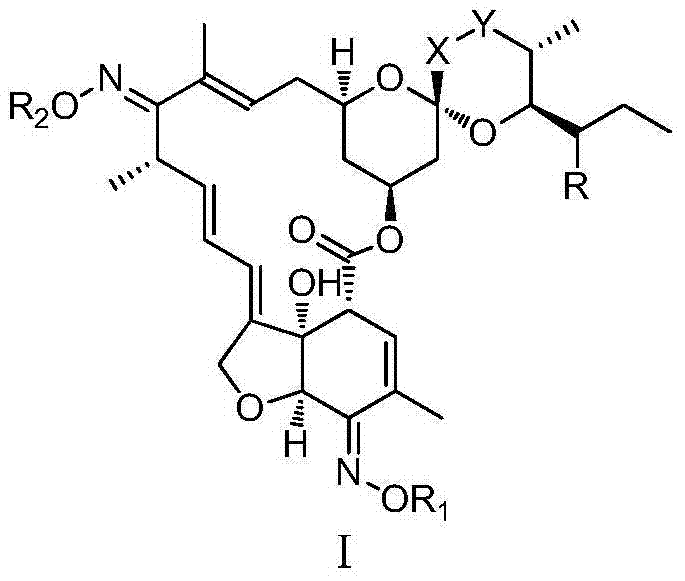
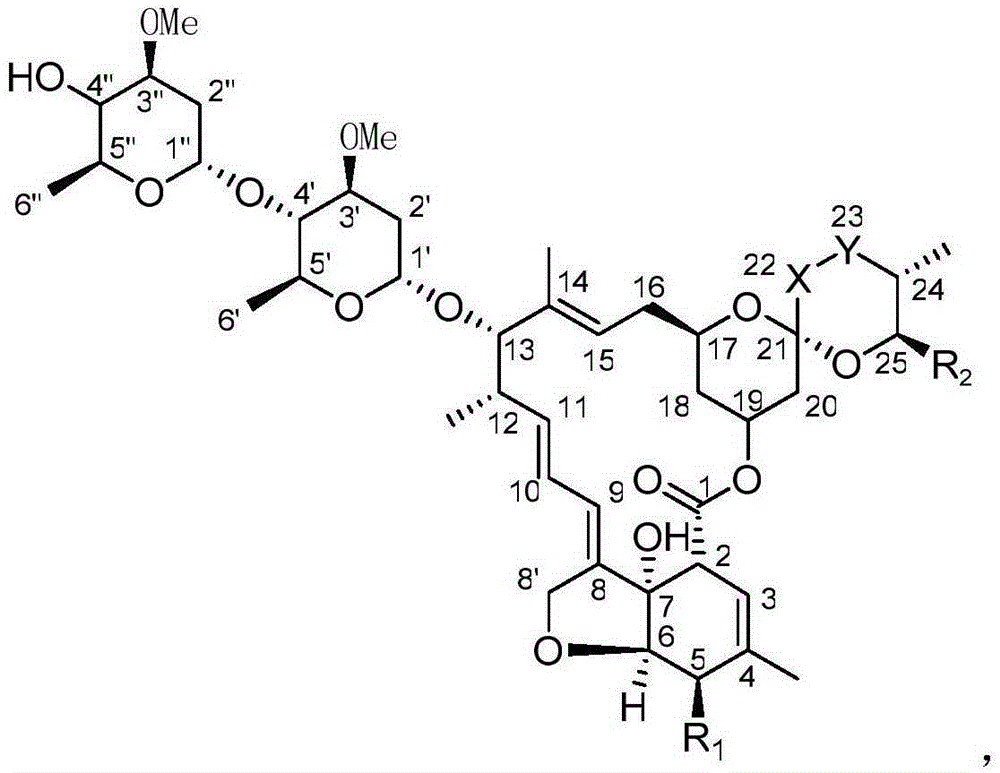
Novel Avermectin derivative and preparation method thereof
ActiveCN103421065AHigh activityImprove efficacySugar derivativesSugar derivatives preparationDistillationHigh activity
The invention relates to a novel Avermectin derivative and a preparation method thereof. The preparation method comprises the following steps: A, dissolving Avermectin B1a or Avermectin B2a into an organic solvent; B, cooling down the solution, adding a substitution reagent and dripping an acid-capturer; C, adding the reaction solution into a sulfuric acid solution, regulating pH, stilling, layering, extracting, adding an organic phase into an ammonia solution, regulating pH to be neutral, stilling, layering, extracting and merging the organic phase; D, performing distillation of the organic phase under reduced pressure to obtain the product. According to the invention, on the basis of the Avermectin B2a or B1a, C5-OH is subject to further chemical modification, and compared with the common Avermectin product, the novel Avermectin derivative has the advantages of high drug effect, broad insecticidal spectrum, good persistence, no residues, no pollution, high efficiency and no resistance. The novel Avermectin derivative has extremely high activity on many pests and mite, has the function of not only stomach toxicity but also killing and has a very good insecticide effect at a very low dosage.
Owner:DAQING JEFENE BIO CHEM
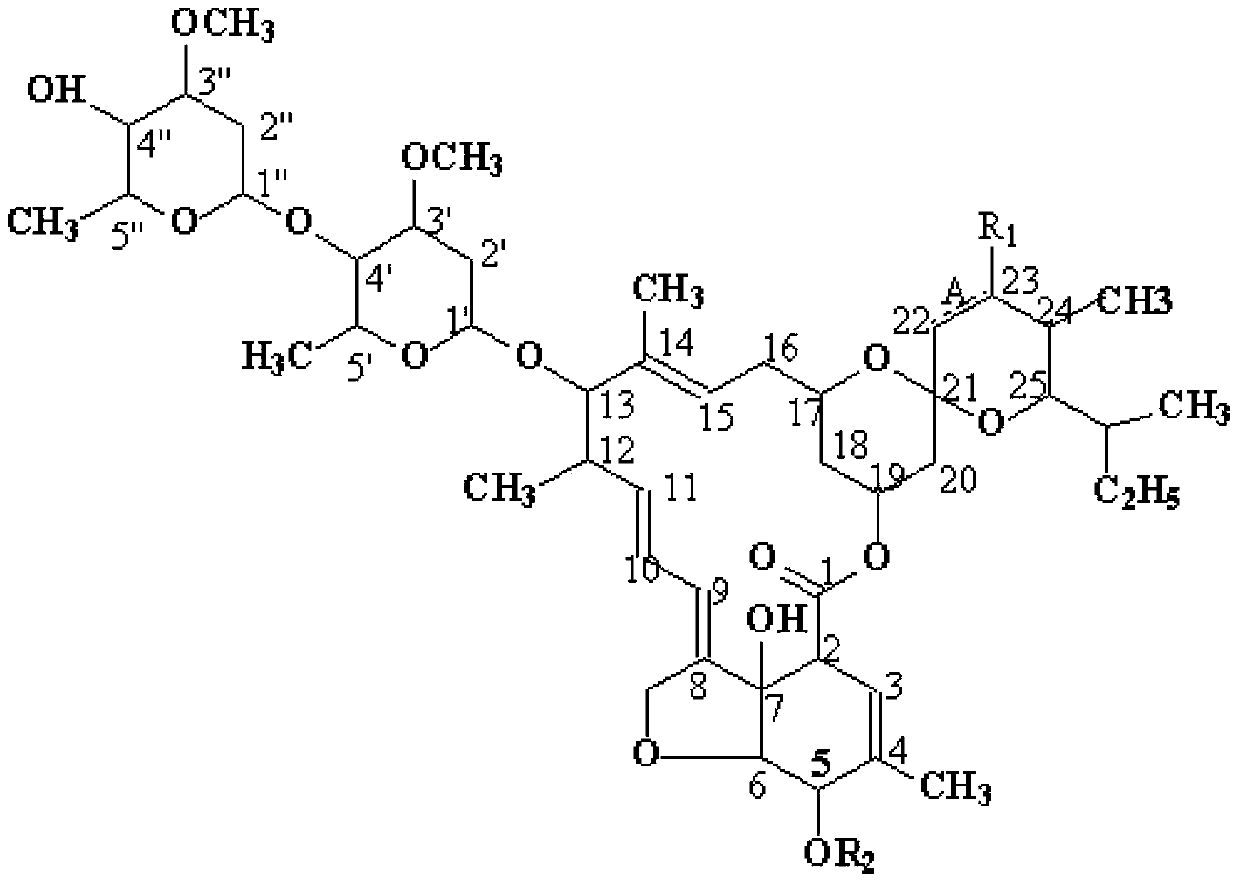
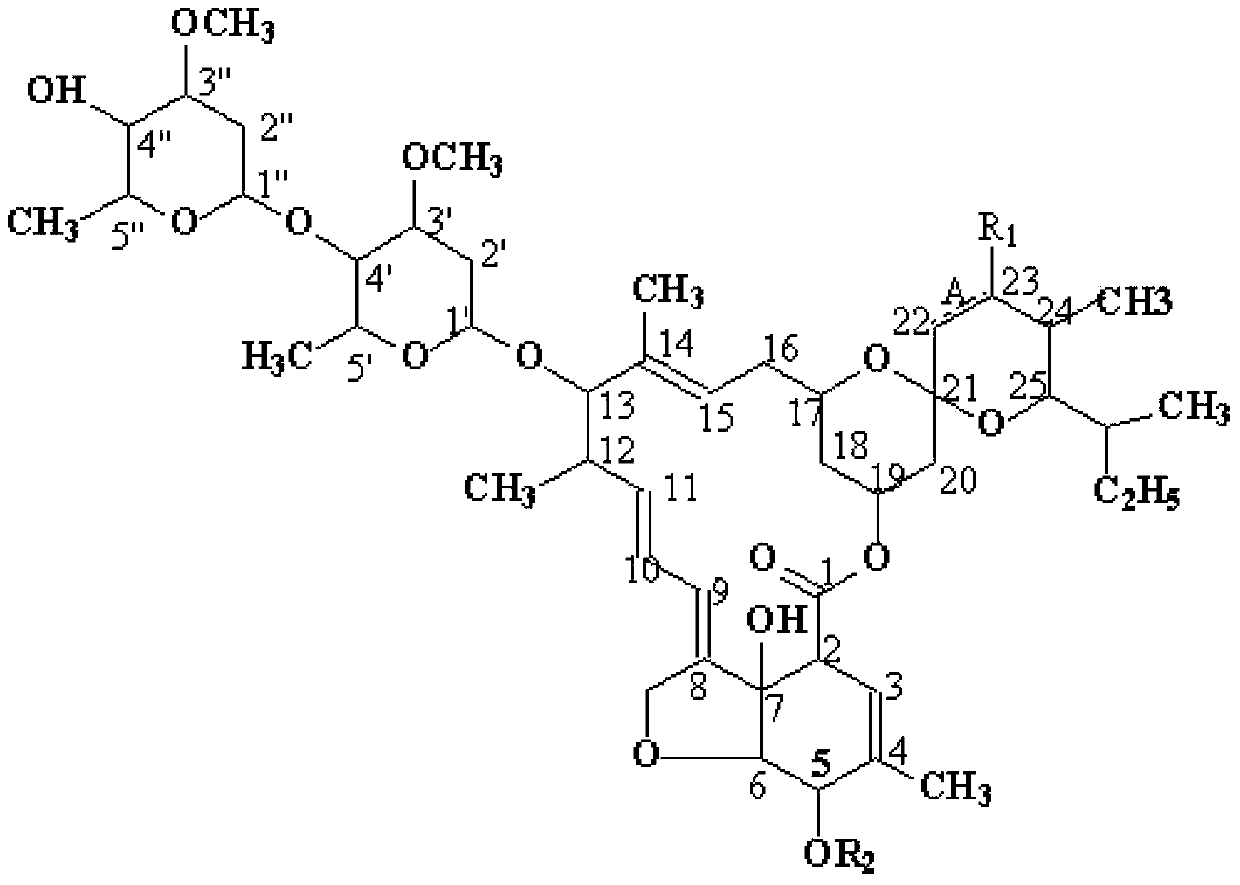
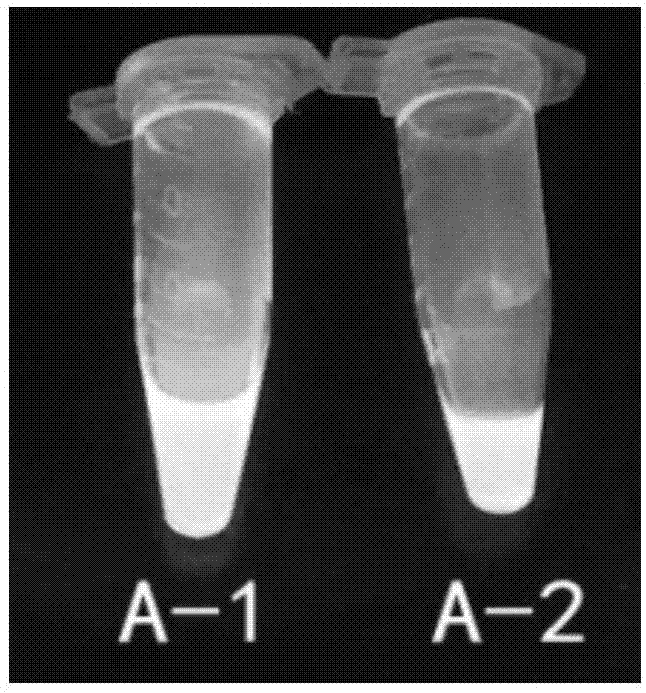
Abamectin B1a fluorescence indicator and applications thereof
InactiveCN103788159AGood prospects for pharmacological applicationOvercome harmOrganic active ingredientsBiocideAvermectin B1aAnticarcinogen
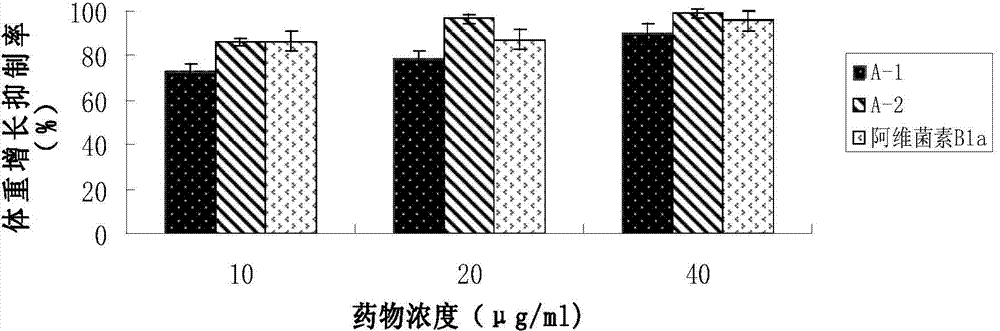
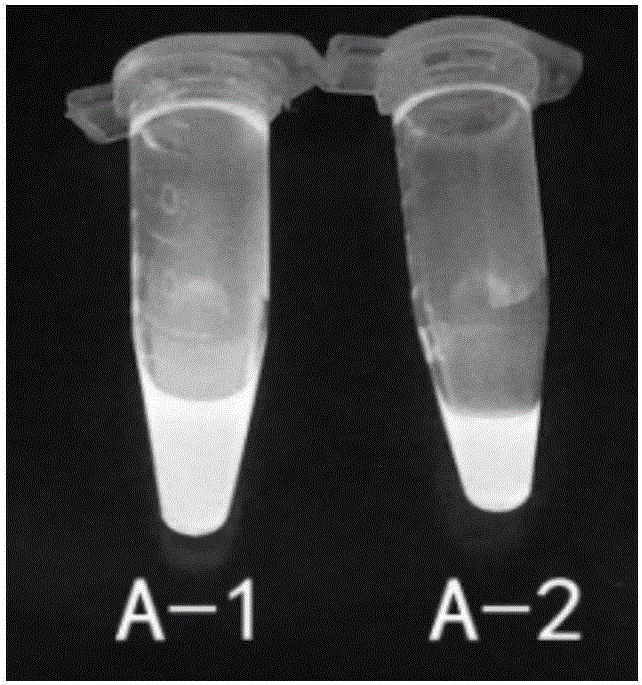
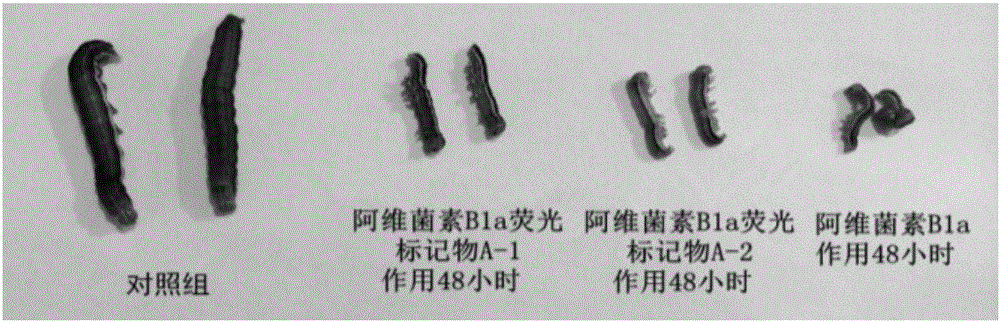
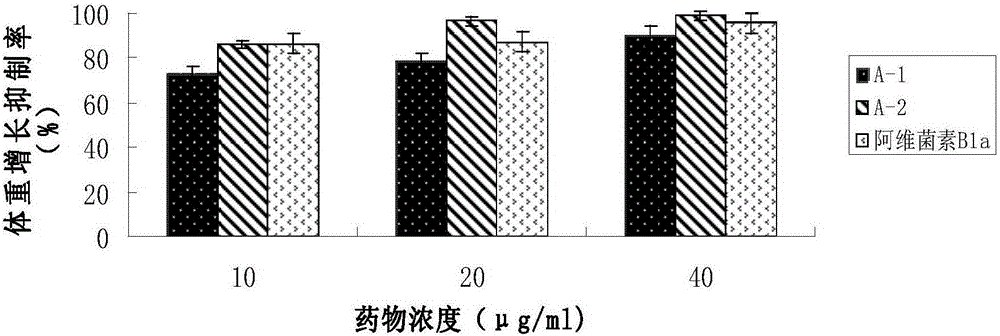
The invention discloses an abamectin B1a fluorescence indicator, which is a compound shown in a formula A-1 or A-2 in the specification. The abamectin B1a fluorescence indicator can be used as a pesticide, an insect physiological indicator and an insect cell fluorescence tracer, and can also be used as an anticancer agent.
Owner:EAST CHINA UNIV OF SCI & TECH
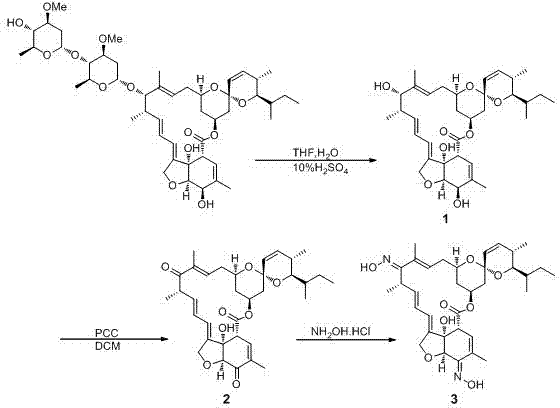
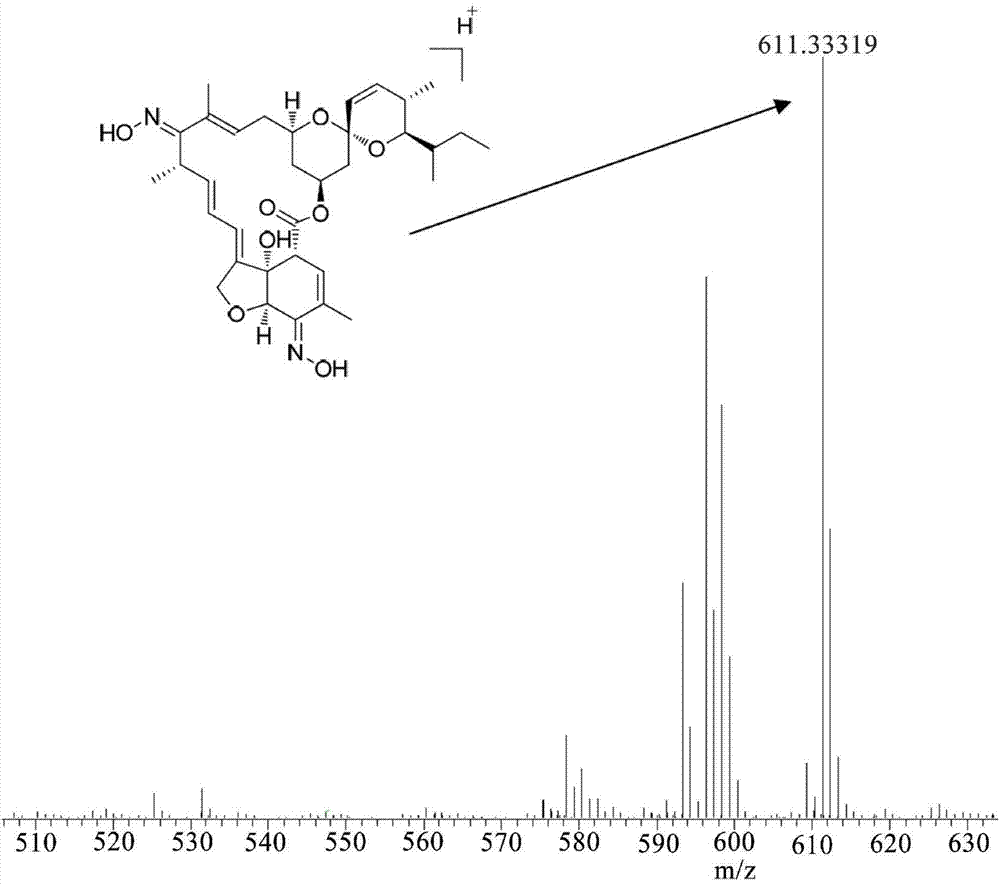
Synthetic method of emamectin benzoate crude product
ActiveCN110563784AReduce contentIncrease contentSugar derivativesSugar derivatives preparationAvermectin B1aAbamectin
The invention relates to a synthetic method of an emamectin benzoate crude product, which is characterized in that: avermectin B1a is used as an initial raw material, and is subjected to an oxidationreaction, an ammoniation reaction, a reduction reaction and a salification reaction to obtain the emamectin benzoate crude product, and the reactions are carried out in a reaction system using dimethylformamide, tetrahydrofuran or tetrahydropyrane as a reaction solvent. According to the invention, the abamectin B1a is directly used as an initial synthesis raw material; the emamectin benzoate is synthesized by adopting a four-step method instead of a six-step method, so that the synthesis process steps are simplified, the production period is shortened, the production cost advantage is obvious,only one reaction solvent is used in the whole synthesis process, the recycling is convenient, the discharge amount of byproducts is very low, and the environmental pollution is not easily caused.
Owner:NINGXIA TAIYICIN BIOTECH CO LTD
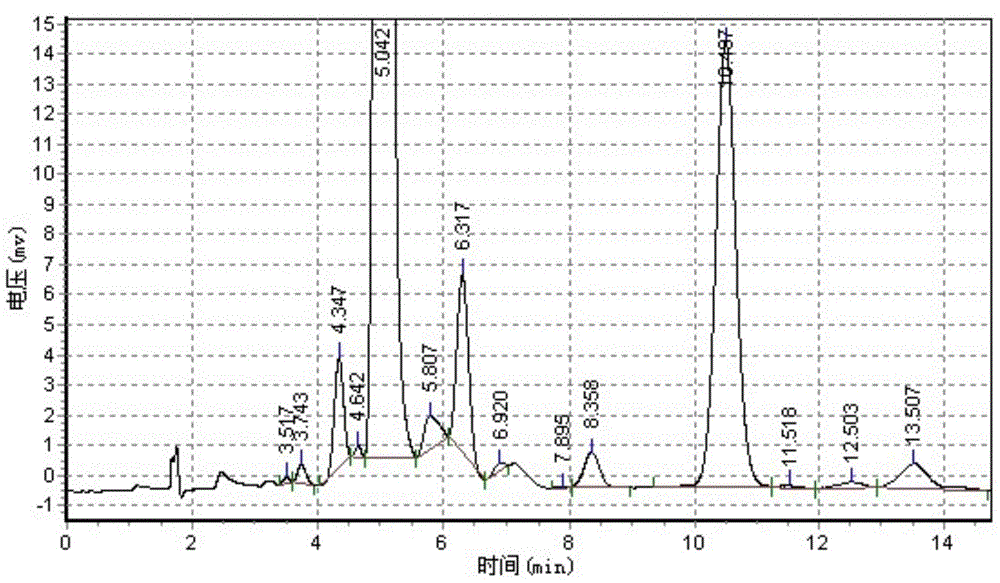
Quick analysis method for reaction process of emamectin benzoate
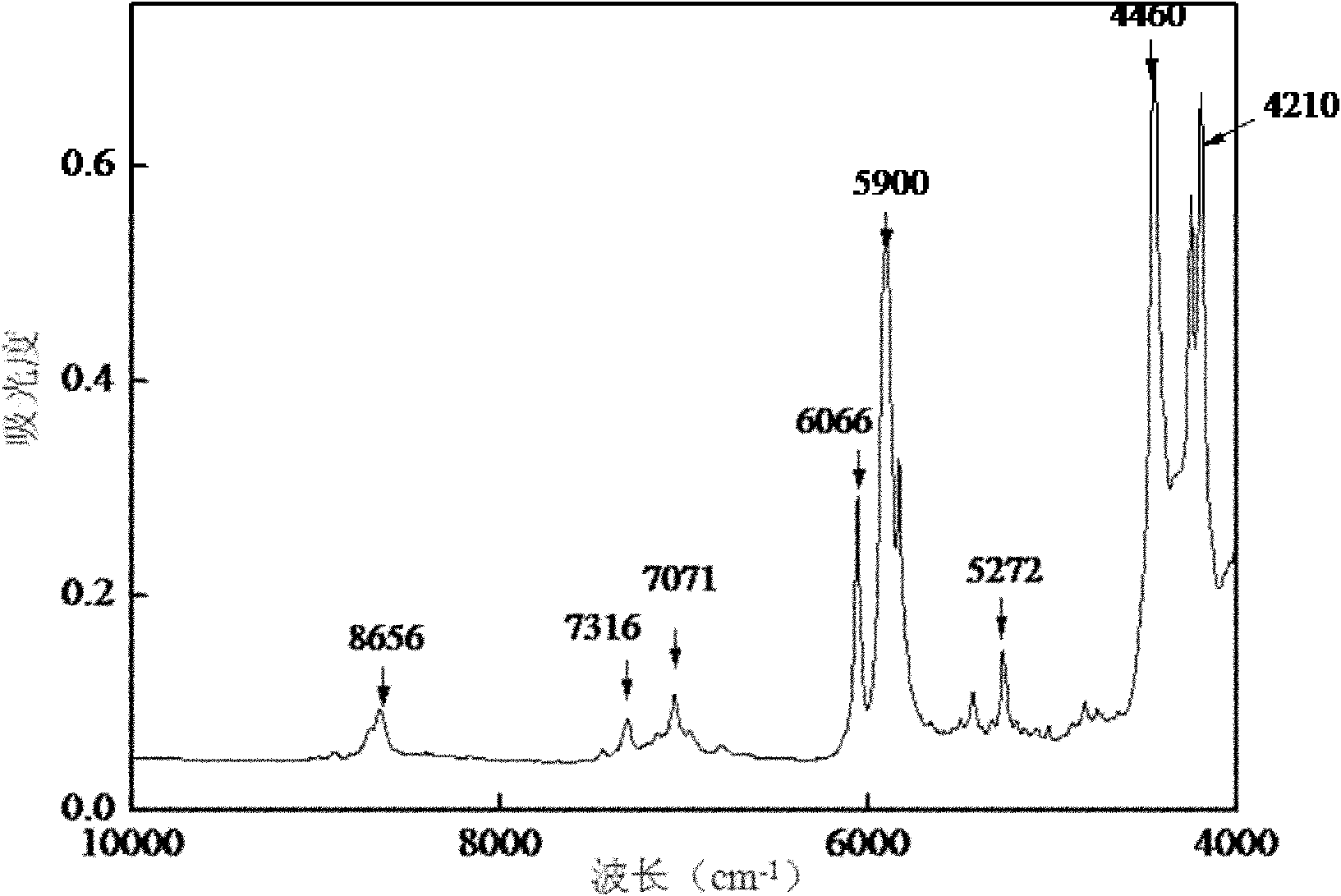
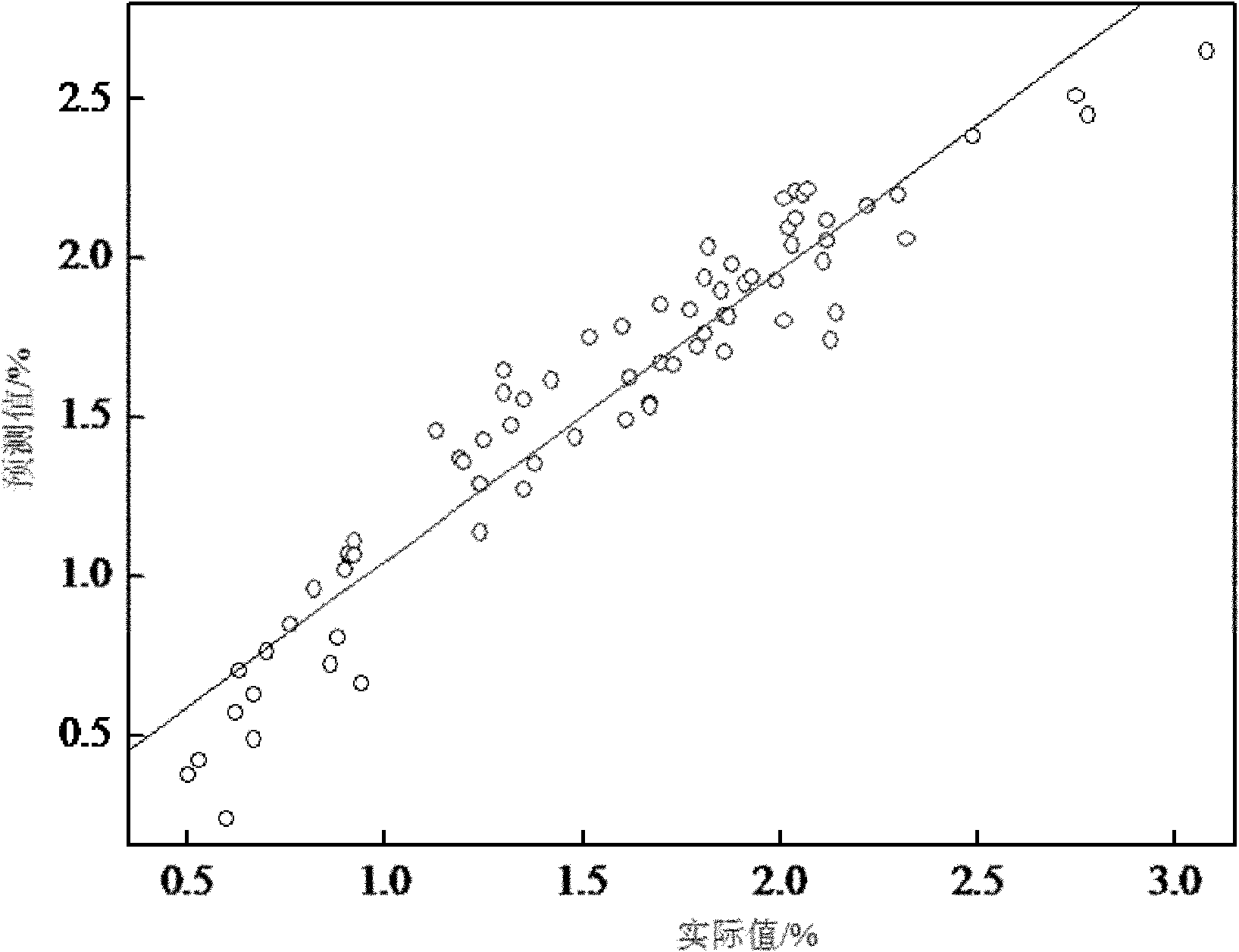
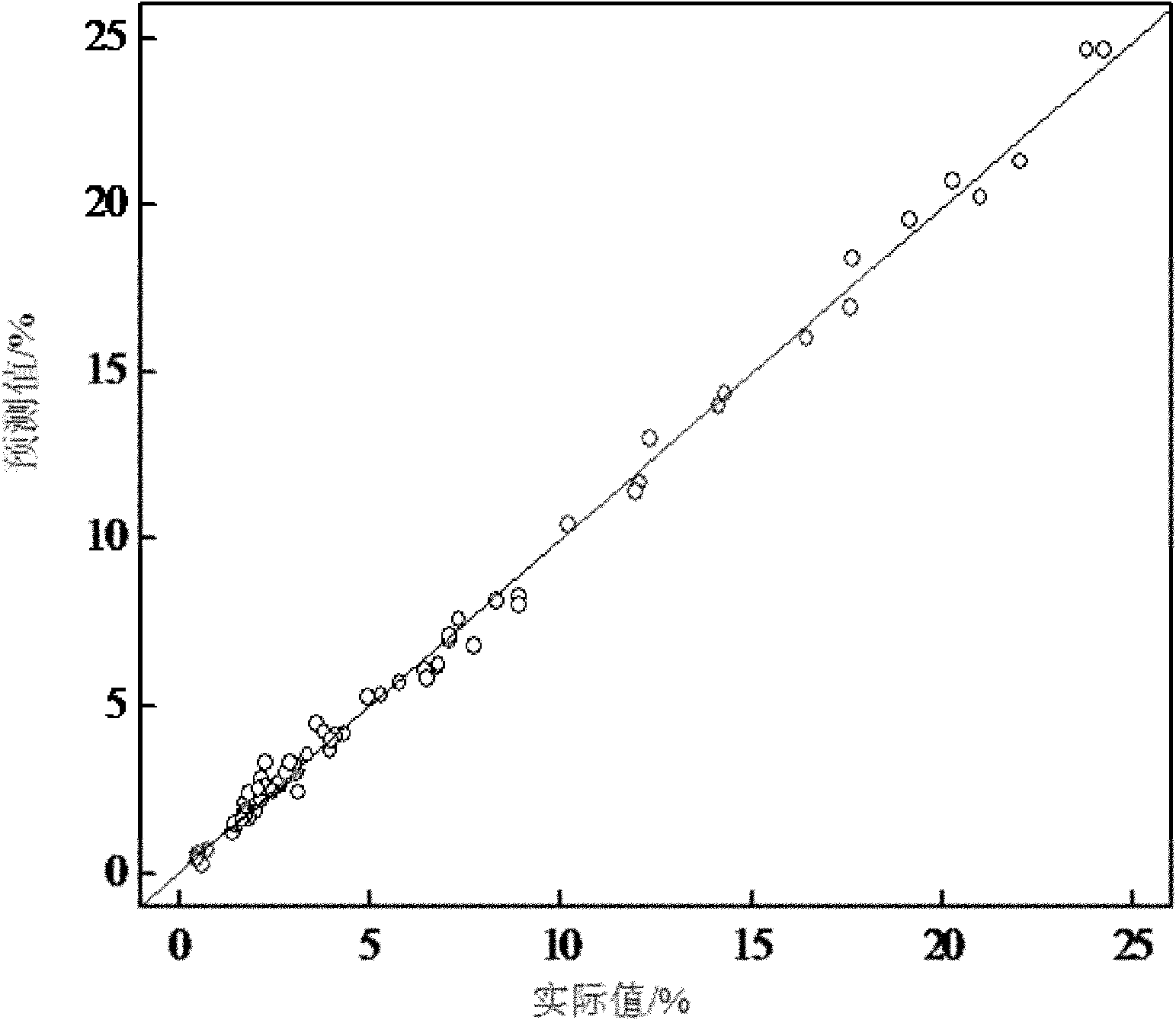
ActiveCN102042966ANon-destructivePersonnel hazardColor/spectral properties measurementsSpectral patternChemical reaction
The invention discloses a quick analysis method for a reaction process of emamectin benzoate and relates to an analysis method for a chemical reaction intermediate process. By utilizing the method, the problems that the time for high performance liquid chromatograph is long, the operation steps are minute and complicated, the detection is time-consuming, a large number of samples need to be measured, the provided experimental data often lags behind the production process, the monitoring of the production process is incomplete and problems can not be found in time are solved. The method comprises the following steps: 1) establishing a sample library; 2) establishing a sample spectral pattern library; 3) selecting a sample; 4) carrying out pretreatment on the sample; 5) establishing a standard sample model; 6) establishing a quantitative analysis model, and getting an optimal quantitative analysis model; and 7) collecting spectral information of the sample to be tested, and predicting the contents of residual avermectin B1a and double-protection matter in the sample to be tested through the optimal quantitative analysis model. The analysis of the sample can be completed within 1 minute by the method, thus the method is time-saving, labor-saving and cost-saving and is easy for operation.
Owner:HARBIN INST OF TECH
Method for synthesizing milbemycin oxime compound
InactiveCN106565740AHigh yieldMild reaction conditionsOrganic chemistryBulk chemical productionChemical synthesisAvermectin B1a
The object of the invention is to provide a method for synthesizing a milbemycin oxime drug. The implementation method provided by the invention comprises the steps: subjecting one or the mixture of avermectin B1a and avermectin B1b, which serves as a raw material, to a hydroxyl protecting reaction so as to protect a hydroxyl group at the C5 position; then, carrying out hydrolysis so as to remove a glycosyl group at the C13 position; then, carrying out a reaction so as to remove a hydroxyl group at the C13 position; and then, removing a hydroxyl protection group at the C5 position, carrying out hydroxyl oxidation so as to produce keto-carbonyl, and carrying out an oximation reaction, thereby finally obtaining a milbemycin oxime compound. According to the method, the milbemycin oxime compound product is synthesized from avermectins, which serve as the raw material, by a chemical synthesis method; and the chemical synthesis method has mature route and has no need of taking milbemycin, which is required to be obtained through fermentation, as the raw material, so that the cost and scarcity of the raw material are reduced, the large-scale industrial production is better facilitated, milbemycin oxime products are enriched, and thus, the shortage of domestic pet parasite treatment drugs is made up.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Method for determining abamectin in soil
The invention relates to a method for determining abamectin in soil, which comprises the following steps: adding a soil sample into acetone for treatment, and extracting a sample solution to be detected; preparing an abamectin standard solution by using acetonitrile as a solvent; respectively detecting and analyzing the sample solution to be detected and the abamectin standard solution by adoptinga high performance liquid chromatography; and carrying out qualitative and quantitative analysis on the abamectin B1a and the abamectin B1b in the soil sample according to the detection and analysisresults. The method for determining abamectin in soil provided by the invention has the advantages of simple and convenient detection process, high sensitivity and low detection limit, which can realize the rapid detection of the abamectin content in an actual soil sample, and has good application prospect.
Owner:SHANGHAI SEP ANALYTICAL SERVICES CO LTD
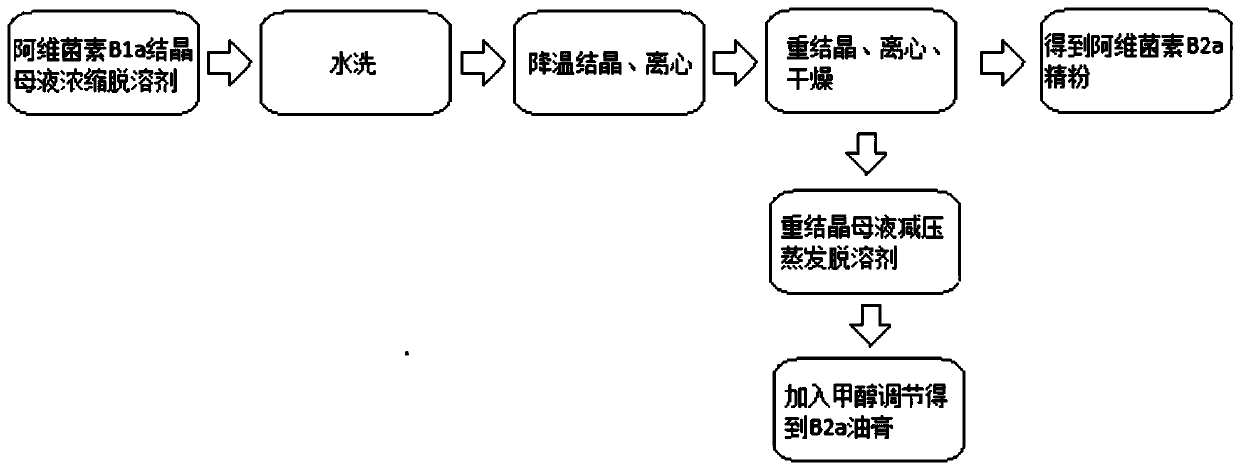
Method capable of simultaneously preparing avermectin B2a fine powder and ointment
The invention discloses a method capable of simultaneously preparing avermectin B2a fine powder and ointment. The method includes the steps of avermectin B1a crystallized mother liquor concentrating and solvent removal, water washing, cooling crystallization, centrifugation, avermectin B2a fine powder preparation, recrystallized mother liquor vacuum evaporation and solvent removal, methanol addingfor adjustment to obtain B2a ointment and the like. According to the method, by studying the B2a component, screening solvents and optimizing the production process, the B2a component fine powder isextracted and the B2a ointment is obtained; due to the fact that the B2a component is extracted from a B1a extraction by-product, the method can expand the avermectin industrial chain.
Owner:江苏物网慧农科技集团有限公司 +1
Extracting method for abamectin B1a
ActiveCN106632552AReduce lossImprove leaching rateSugar derivativesSugar derivatives preparationFiltration membraneAbamectin
The invention relates to an extracting method for abamectin B1a. The extracting method is characterized by comprising the following process steps: first, filtering abamectin fermenting liquid by adopting a micro-filtration membrane to obtain abamectin-rich membrane paste; then, extracting the membrane past by using a compound solvent; next, adsorbing by using an aluminium oxide chromatographic column and desorbing by butyl alcohol after the obtained extracting liquid is concentrated; collecting desorbing liquid of a B1 stage, de-colouring, filtering and performing primary crystallizing to obtain abamectin B1 crude product; re-crystallizing through ethyl acetate to obtain a finished product with high content of the abamectin B1a. According to the extracting method, the abamectin is extracted in the mode of adopting micro-filtration membrane and compound solvent extracting and combining an aluminium oxide chromatographic column and butyl alcohol desorbing process, so that the abamectin and the product quality can be effectively improved; moreover, the process is simple; the solvent loss is little; the solvent is easy to recycle; the safety is high; meanwhile, environmental pollution can be reduced.
Owner:NINGXIA TAIYICIN BIOTECH CO LTD
Streptomyces avermitilis synthetic medium and preparation method of streptomyces avermitilis fermentation broth
InactiveCN107338210ASuitable for growthClear ingredientsBacteriaMicroorganism based processesAbamectinDipotassium phosphate
The invention discloses a streptomyces avermitilis synthetic medium. The streptomyces avermitilis synthetic medium comprises the following raw materials by weight according to 1L volume: 8-25 g of maltose monohydrate, 3-14 g of anhydrous dextrose, 0.8-2.5 g of threonine, 0.3-0.5 g of magnesium sulfate heptahydrate, 0.005-0.015 g of iron vitriol, 0.15-0.45 g of dipotassium phosphate trihydrate, 0.01-0.02 g of manganese sulfate monohydrate, 0.3-0.6 g of sodium chloride, 4-6 g of 3-(4-morpholino)propanesulphonic acid and the balance of water. The invention discloses a preparation method of a streptomyces avermitilis fermentation broth. The streptomyces avermitilis synthetic medium is reasonable in formula and rich in nutrition, meets the growth and avermectins production requirements of streptomyces avermitilis, and is suitable for related basic scientific study, and the abamectin B1a effective component is 2.84 times of that of a clear medium.
Owner:TIANJIN UNIV OF SCI & TECH +1
Fermentation method for increasing content of abamectin B1a
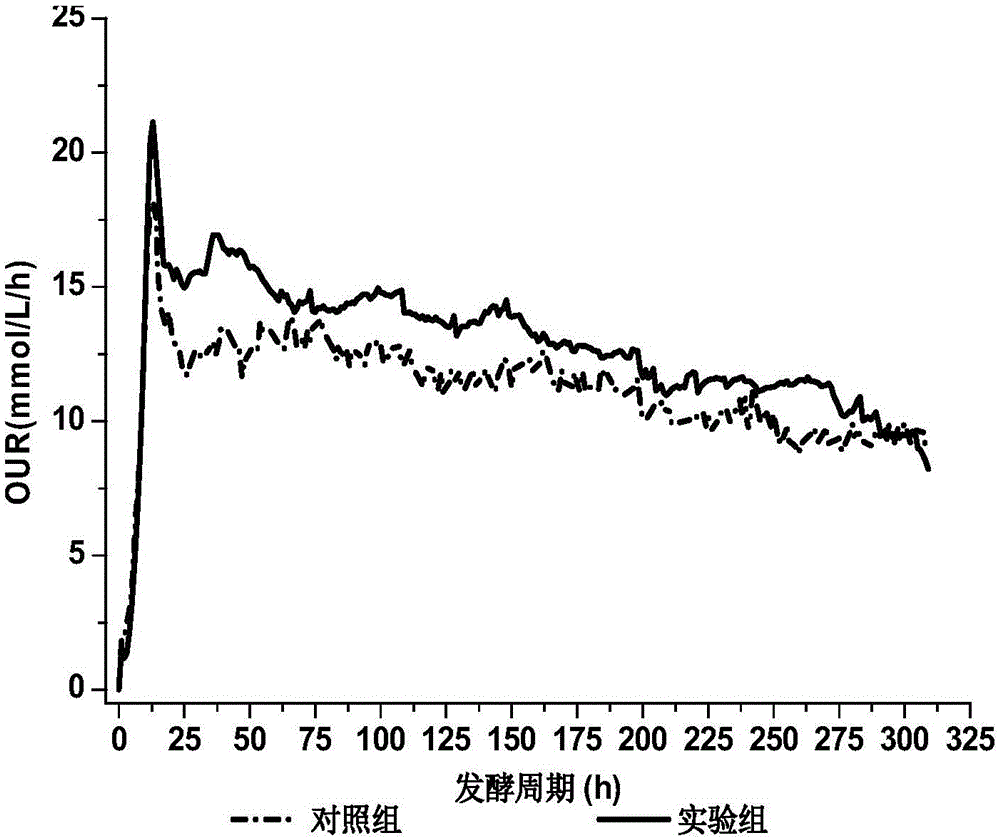
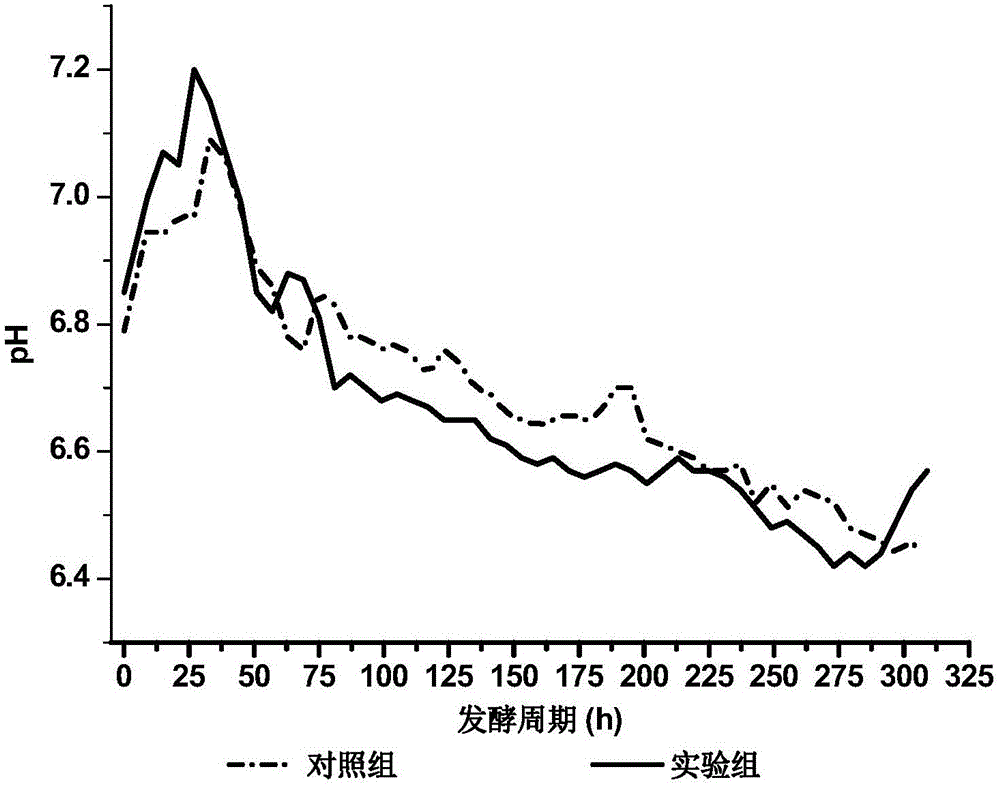
PendingCN110551784AImprove fermentation titerPromote fermentationMicroorganism based processesFermentationAvermectin B1aFermentation broth
The invention relates to a fermentation method for increasing the content of abamectin B1a. The fermentation method comprises the step: carrying out seed culture and fermentation culture on streptomyces avermitilis serving as a fermentation strain. The fermentation method is characterized in that an oxygen carrier is added in a fermentation culture process. The oxygen carrier and a fermentation solution are not mutually dissolved by adding the oxygen carrier n-dodecane not serving as a carbon source when the abamectin is fermented for 12-20 h, so that the direct oxygen transfer resistance of agas phase and a liquid phase is reduced to the great extent, the oxygen dissolution capacity of a substrate is improved, the fermentation potency of the abamectin can be kept at 7000 mu / ml-8000 mu / ml, and the content of the abamectin B1a can be 96%-98%. The fermentation method is simple and effective in process, can be applied to industrial production of abamectin and has a certain guiding significance for the optimization of an abamectin fermentation process.
Owner:NINGXIA TAIYICIN BIOTECH CO LTD
Method for improving yield of avermectin and producing strain
ActiveCN108823137AIncrease productionRaise the fermentation unitBacteriaTransferasesAvermectin B1aMicrobiology
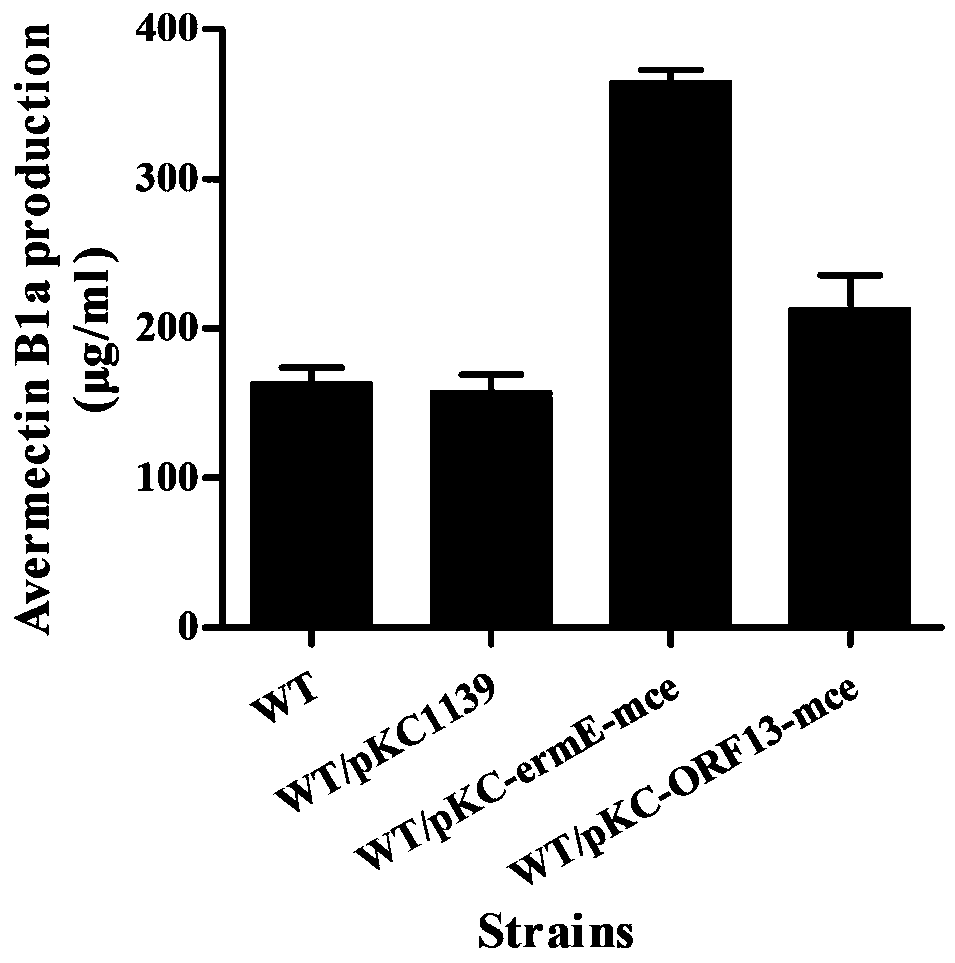
The invention provides a method for improving the yield of avermectin and a producing strain. The method is to construct an overexpressed genetically engineered strain of a gene mce (SAV2857) encodedby a methylmalonyl-CoA epimerase in streptomyces avermitilis. The genetically engineered bacterium obtained by the invention can be directly used for the fermentation production of the avermectin; thefermentation unit of the avermectin is improved, the yield of avermectin B1a is increased by about 25% compared with that of an original strain, and the production cost is lowered; and the genetically engineered bacterium has important industrial application prospects.
Owner:CHINA AGRI UNIV
Abamectin b1a fluorescent marker and its application
The invention discloses an abamectin B1a fluorescence indicator, which is a compound shown in a formula A-1 or A-2 in the specification. The abamectin B1a fluorescence indicator can be used as a pesticide, an insect physiological indicator and an insect cell fluorescence tracer, and can also be used as an anticancer agent.
Owner:EAST CHINA UNIV OF SCI & TECH
Extraction method for improving output and internal quality of avermectin B1a fine powder
ActiveCN106380500AIncrease productionReduce the number of recrystallizationSugar derivativesSugar derivatives preparationSolubilityAvermectin B1a
The invention relates to an extraction method for improving the output and internal quality of avermectin B1a fine powder. The method comprises the following steps: adding hyphae of avermectin into a methanol solvent with a temperature of 40 to 45 DEG C and a mass concentration of 95 to 97%, carrying out extraction, then cooling to a temperature lower than 0 DEG C, maintaining the temperature for 2 to 4 hours, concentrating and purifying filtrate, and finally carrying out crystallization and recrystallization to obtain the avermectin B1a crystals. The hyphae of avermectin is added into methanol with a temperature of 40 to 45 DEG C and a mass concentration of 95 to 97% to obtain an extract; as the extract cools down, the solubility of higher fatty acid grease in the methanol is reduced quickly, the higher fatty acid grease is precipitated, after filtering, the crystallization and recrystallization will remove grease residues from the fine powder, thus the content of avermectin B1a is increased, and the internal quality of fine powder is improved therefore.
Owner:QILU PHARMA INNER MONGOLIA
Crystallization method of abamectin Bla
The present invention relates to a crystallization method of abamectin B 1a. Said method includes the following steps: using crystallization solvent n-butanol to stir and dissolve primary crude powder of abamectin B 1a at 75-100deg.C to saturation, filtering while the saturated solution is hot to obtain clear hot-saturated solution; slowly cooling said solution to that when the supersaturation degree is 1-3, adding crystal seeds, constant stirring for 20-60min, its stirring speed is 120-300rpm, and cooling to make crystallization, fitering crystal slurry or centrifugally-separating said crystal slurry, washing crystal and drying so as to obtain the invented abamectin B 1a.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
A kind of avermectin producing bacteria and its preparation method and application
The invention relates to avermectin producing bacteria and a preparation method and application thereof. Specifically, aveD gene in wild type avermectin producing bacteria is inactivated to obtain a strain getting rid of an A component and only producing a B component; by anaplerosis of a homologous gene of the aveC gene (preferably meiC gene in a Meilingmycin biosynthetic gene cluster) on the basis of simultaneous inactivation the aveD gene and aveC gene, 95wt% above of the obtained mutant fermentation product is avermectin B2a. The product avermectin B2a can be used as a precursor for being directly used for large-scale synthesis of high purity avermectin B1a and ivermectin.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
A kind of preparation method of Abamectin b2a
ActiveCN104650167BHazard reductionReduce pollutionSugar derivativesSugar derivatives preparationAvermectin B1aFiltration
The invention relates to a preparation method of high-purity abamectin B2a, which comprises the steps of concentrating the abamectin B1a crystallization mother liquor under vacuum conditions to no fraction to obtain a thick ointment; adding an extractant for extraction to obtain an extract , and then use saturated brine to wash the extract for 2 to 3 times, remove the water phase to obtain n-butyl acetate solution; cool the obtained n-butyl acetate solution to crystallize, grow crystals, and suction filter to obtain crude crystals of abamectin B2a ; Abamectin B2a coarse crystal recrystallization suction filtration, drying, to obtain high-purity avermectin B2a fine powder. The present invention uses the environment-friendly non-toxic solvent n-butyl acetate to replace the toxic solvent aromatic hydrocarbon to produce abamectin B2a high-quality goods. During the production operation, it causes little harm to the health of on-site employees, and the pollution to the environment is also relatively small. In addition, n-butyl acetate is adopted as B2a as crystallization solvent, and the purity of the obtained abamectin B2a fine powder is above 95%.
Owner:QILU PHARMA INNER MONGOLIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com