Novel Avermectin derivative and preparation method thereof
A technology of abamectin and its derivatives, which is applied in the field of novel abamectin derivatives and its preparation, can solve the problems of small advantages and short duration of action, and achieve quick effect, long duration of effect, and insecticidal spectrum wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
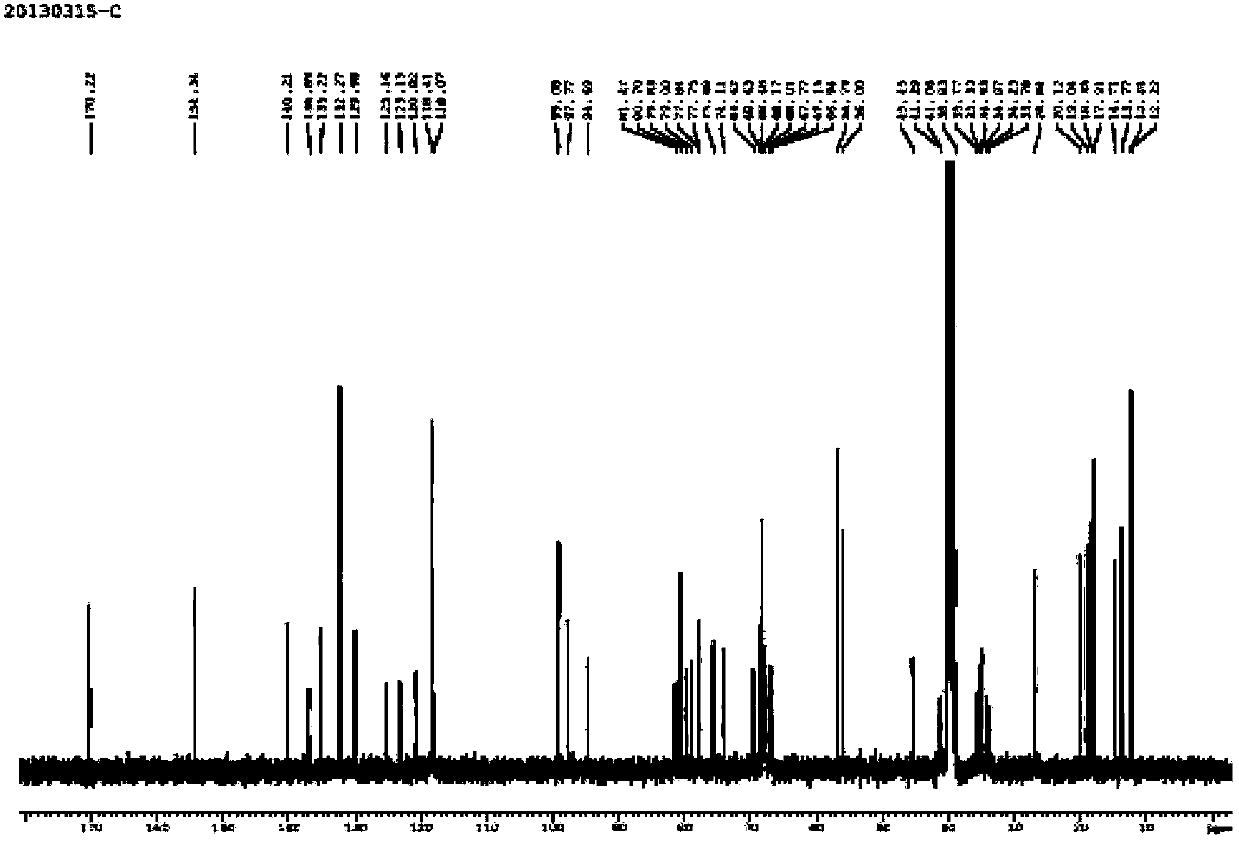
Image
Examples
Embodiment 1
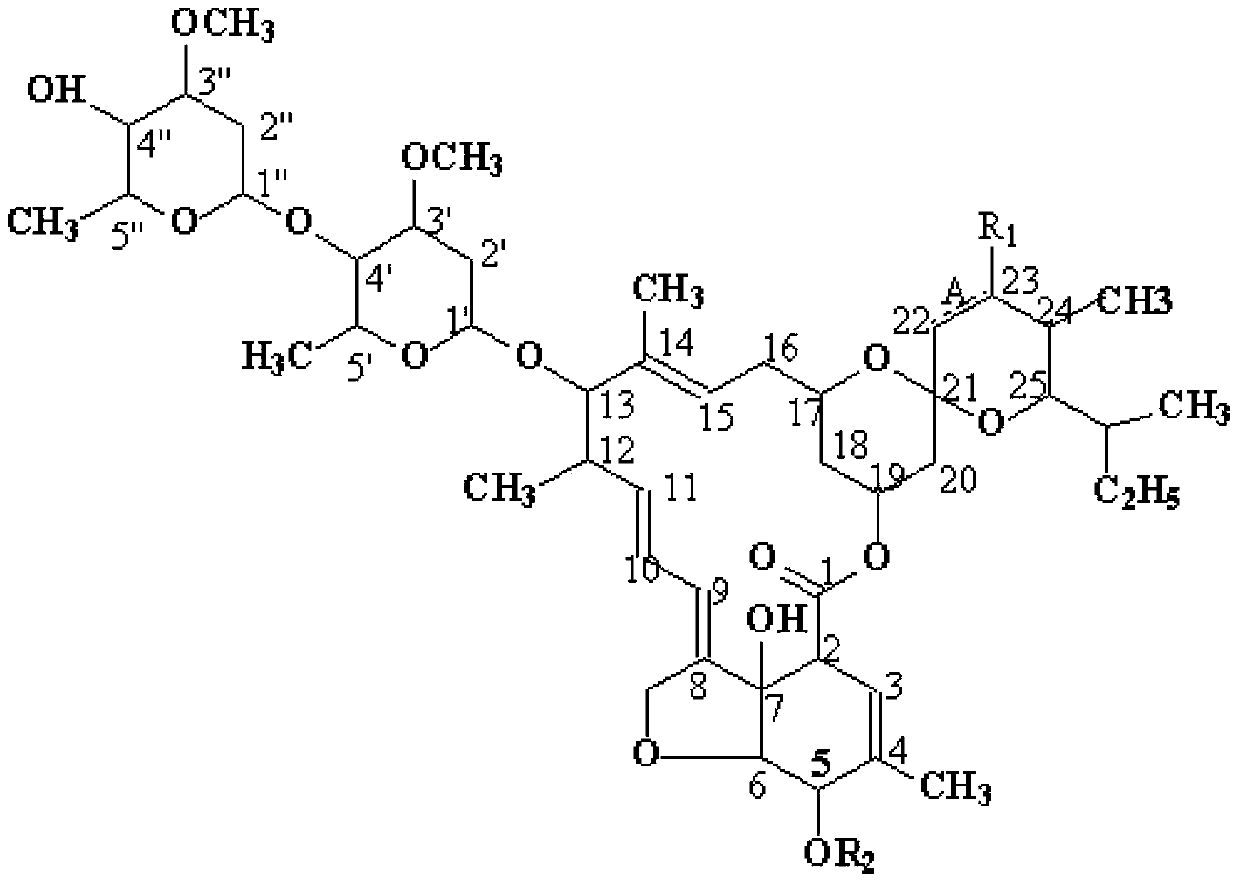
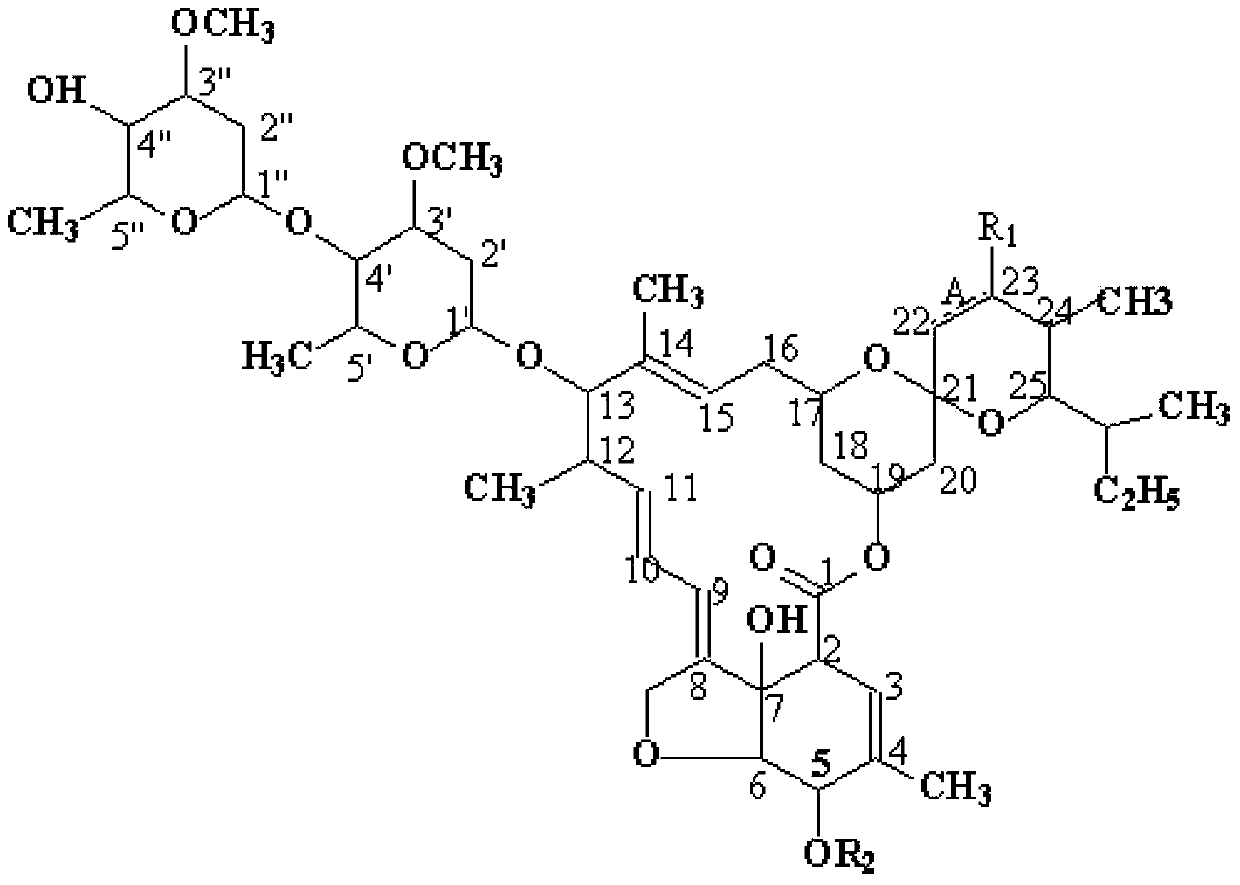
[0027] 1. Synthesis of C 5 -O-Allyl Abamectin B Formic Acid 2a (A is a single bond, R 1 is OH, 5, 22 and 23 are the serial numbers of carbons in the compound structure)
[0028] Reaction formula:
[0029]
[0030] 2. C 5 -O-Allyl Abamectin B Formic Acid 2a The preparation method:
[0031] A. Take 40g, 93.6% Abamectin B 2a Fine powder, dissolved in 200g of organic solvent dichloromethane, dissolved completely.
[0032] B. Cool the above solution to -15°C, add 12g of allyl chloroformate as a substitution reagent, stir for 1 hour, add 5.2g of acid-binding agent tetramethylethylenediamine dropwise, and control the reaction temperature in the range of -10~-5°C After the dropwise addition was completed, stirring was continued for 2 hours.
[0033] C. Add the reaction solution to 200mL sulfuric acid solution with a mass fraction of 2%, adjust the pH to 1-2 with sulfuric acid with a mass fraction of 98% under stirring, let stand to separate layers, and extract the water lay...
Embodiment 2
[0036] 1. Synthesis of C 5 -O-tert-butyldimethylsilyl abamectin B 2a (A is a single bond, R 1 is OH, 5, 22 and 23 represent the serial number of carbon in the compound structure)
[0037] Reaction formula:
[0038]
[0039] 2. C 5 -O-tert-butyldimethylsilyl abamectin B 2a preparation method
[0040] A. Take 40g, 93.6% Abamectin B 2a Fine powder, dissolved in 220g of organic solvent dichloroethane, dissolved completely.
[0041] B. Cool down the above solution to -15°C, add 10.2g of substitution reagent tert-butyldimethylsilyl chloride, stir for 1 hour, add 4.4g of acid-binding agent triethylamine dropwise, control the reaction temperature at -15~-10°C Within the range, stirring was continued for 2 hours after the dropwise addition was completed.
[0042] C. Add the reaction solution to 200mL, 2% sulfuric acid solution, adjust the pH to 1-2 with sulfuric acid under stirring, let stand to separate layers, extract the water layer once with 20mL dichloromethane, combine...
Embodiment 3
[0045] 1. Synthesis of C 5 -O-Formic acid methyl abamectin B 1a (A is a double bond, R 1 is H, 5, 22 and 23 are the serial numbers of carbons in the compound structure)
[0046] Reaction formula:
[0047]
[0048] 2. C 5 -O-Formic acid methyl abamectin B 1a The preparation method:
[0049] A. Take 40g, 94.3% Abamectin B 1a Fine powder, dissolved in 270g of organic solvent chloroform, dissolved completely.
[0050] B. Cool the above solution to -25°C, add 4.5g of methyl chloroformate as a substitution reagent, stir for 1 hour, then add 5.0g of acid-binding agent tetramethylethylenediamine dropwise, and control the reaction temperature in the range of -20~-15°C After the dropwise addition was completed, stirring was continued for 2 hours.
[0051] C. Add the reaction solution to 200mL, 2% sulfuric acid solution, adjust the pH to 1-2 with sulfuric acid under stirring, let it stand for stratification, extract the water layer with 30mL chloroform once, and add the combined...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com