A kind of preparation method of Abamectin
A technology of abamectin and purpose, applied in the field of high-purity abamectin, can solve the problems of small batch volume, pollute the environment, high cost, etc., and achieve the effects of controllable quality, stable yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of Abamectin crude product:
[0026] The components of slant culture medium, seed culture medium and fermentation medium used in this embodiment are as follows:
[0027] Slant medium: 15g glucose, 3g beef extract, 0.5g asparagine, 0.5g KH2PO4, 19g agar, add tap water to dissolve and adjust the volume to 1000ml, adjust the pH value to 7.2~7.5; sterilize at 121°C for 30min.
[0028] Seed medium: 30.0g cornstarch, 8.0g soybean powder, 10g peanut cake powder, 5.0g yeast powder, 2.0g COCl2, add tap water to dissolve, dilute to 1000 ml, pH value 6.8-6.9, sterilize at 121 ℃ 30 min.
[0029] Fermentation medium: 150.0g corn starch, 30.0g soybean powder, 9.0g yeast powder, (NH4)2SO4 0.25g, COCl2 0.03g, Na2MoO4 0.025g, MnSO4 0.003g, CaCO3 1.0g, amylase 0.03g, add tap water Dissolve and dilute to 1000 ml, pH 7.2-7.4, and sterilize at 121°C for 30 minutes.
[0030] (1) Inoculate the strain of Streptomyces averditilis (No. 192S9) on the slant medium,...
Embodiment 2
[0032] Embodiment 2: Abamectin crude product purification treatment
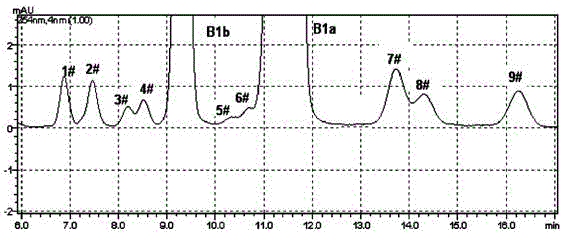
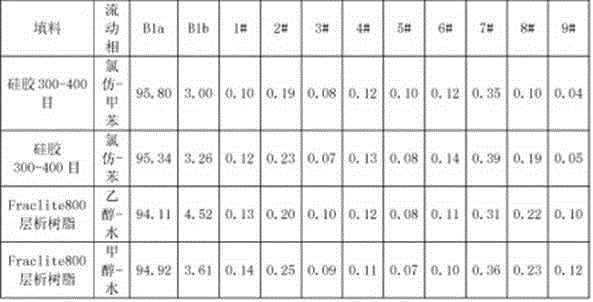
[0033]Get the Abamectin crude product 5g that embodiment 1 prepares, dissolve with 20mL acetone, load the chromatographic column (loading capacity is 500ml) to ODS reverse-phase chromatographic filler, at first use the ethanol aqueous solution (volume concentration 60%) of 10L as Mobile phase elution, and then use 75% ethanol aqueous solution as mobile phase elution (flow rate is 2BV / h), until all abamectins flow out, liquid phase detection 75% ethanol eluent, combined elution according to the detection results Deliquification, concentration, and vacuum drying at 45°C yielded 1.5 g of abamectin B1 (B1a+B1b) with a purity of 99.0% (see Tables 1 and 2 for the proportions of each component).
Embodiment 3
[0034] Embodiment 3: Abamectin crude product purification process
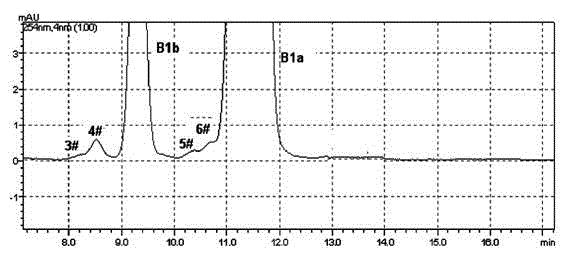
[0035] Get the Abamectin crude product 10g that embodiment 1 prepares, dissolve with 50mL acetone, load the chromatographic column (loading capacity is 1000ml) to ODS reverse-phase chromatographic filler, at first use the methanol aqueous solution (volume concentration 70%) of 15L as Elute with mobile phase, then elute with 85% methanol aqueous solution (flow rate: 4BV / h), until all the abamectin flows out, detect the 85% methanol eluate in liquid phase, combine the eluate according to the detection results, Concentrated and dried under vacuum at 45 °C to obtain 4.1 g of Abamectin B1 (B1a+B1b) with a purity of 99.4% (see figure 2 , The proportion of each component is shown in Table 1 and 2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com