Abamectin B1a fluorescence indicator and applications thereof
A fluorescent marker, abamectin technology, applied in the field of chemical biology, can solve the problems of high cost, limited technical means, no further application, etc., achieves wide application prospect, facilitates chemical biology research, good pharmacology The effect of learning application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of embodiment one 5-abamectin B1a ester (compound A-1)
[0025] Dissolve 6-bromohexanoic acid and sodium azide in DMF, at 80-100°C for 3-6 hours, dilute the reaction mixture with DCM, wash the organic phase with HCl solution, and wash the organic phase with anhydrous sodium sulfate Dry, filter, and spin out the organic phase to obtain 6-azidocaproic acid as a colorless oily compound;
[0026] Dissolve Abamectin B1a in DCM together with imidazole, DMAP, TBDMS-Cl, react at room temperature for 5-8 hours, add water to the reaction solution, extract the reaction solution with DCM, and use saturated chlorination for the organic phase The sodium solution was washed and separated, and the organic phase was dried with anhydrous sodium sulfate, filtered and distilled to obtain the compound avermectin B1a-TBDMS with the 5-hydroxyl protected.
[0027] The prepared compound 6-azidohexanoic acid and abamectin B 1a -TBDMS undergoes esterification reaction under DCC and ...
Embodiment 2
[0034] The preparation of embodiment two 4''-abamectin B1a ester (compound A-2)
[0035] Compound 6-azidocaproic acid and avermectin B prepared in embodiment one 1a Carry out esterification reaction under DCC, DMAP condition, obtain white solid compound Abamectin B1a-N 3 -2; then abamectin B 1a -N 3 -2 and the compound 4-ethynyl-1,8-naphthalimide obtained in Example 1 were dissolved in DMSO: water: tert-butanol (V:V:V=1:1:1), and click chemistry reaction to obtain the final compound fluorescently labeled Abamectin B 1a Compound A-2; wherein the reaction conditions and separation conditions are the same as in Example 1. The target compound A-2 was tested by TOF-MS-ES+, 1 H NMR, 13 C NMR for confirmation. Materialized data:
[0036] 1 H NMR (500MHz, DMSO-d 6 ):δ9.03(d,1H),8.77(s,1H),8.39(t,3H),7.98(d,1H),7.77(t,1H),5.68(br,d,2H),5.60( d,1H),5.53(m,1H),5.47(s,1H),5.43(dd,2H),5.38(s,1H),5.25(d,1H),5.13(s,1H),5.02(t ,1H),4.96(d,1H),4.75(m,1H),4.59(s,1H),4.43(t,3H),4.32(...
Embodiment 3
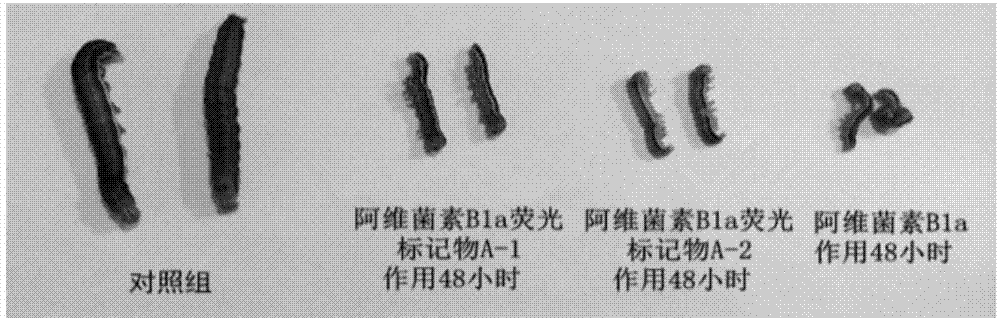
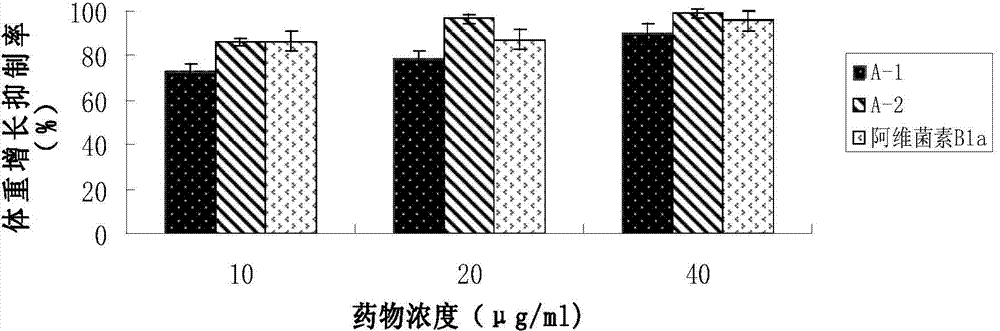
[0039] The measurement of the fluorescent characteristic of embodiment three Abamectin B1a fluorescent markers
[0040] Weighed 1.0 mg of compounds A-1 and A-2 respectively, dissolved them in DMSO, diluted them with PBS buffer to a concentration of 20 μg / ml, and measured the fluorescence characteristics of the fluorescent markers using a PTI steady-state fluorometer.
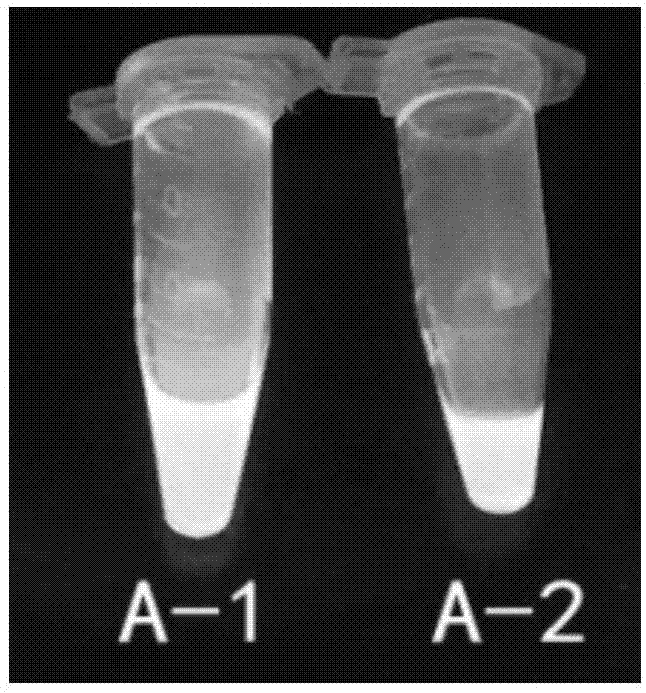
[0041] The results showed that the maximum excitation wavelength of A-1 was 375nm and the emission wavelength was 480nm; the maximum excitation wavelength of A-2 was 370nm and the emission wavelength was 475nm. After A-1 and A-2 were dissolved in DMSO, bright blue fluorescence could be observed under 365nm ultraviolet light. figure 1 Show Abamectin B 1a Fluorescent characteristics of fluorescent markers A1 and A2 under the excitation wavelength of 365nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com