Quick analysis method for reaction process of emamectin benzoate
A technology of methylamino abamectin and benzoate, which is applied in the analysis field of the intermediate process of chemical reaction, can solve the problems of incomplete monitoring of production process, long high-performance liquid chromatography analysis time, and inability to detect in time, and the like, To achieve the effect of easy operation, saving raw materials and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
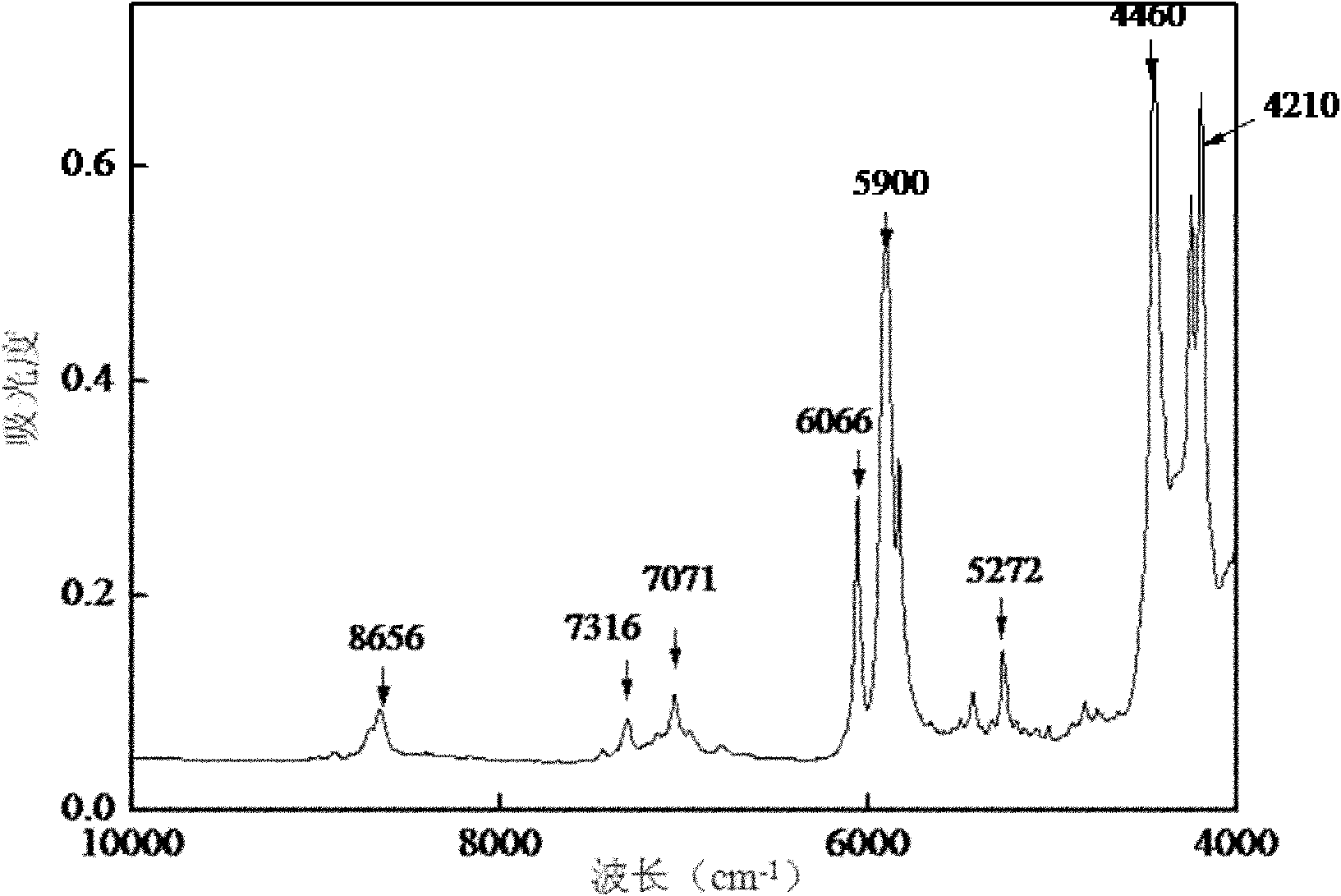
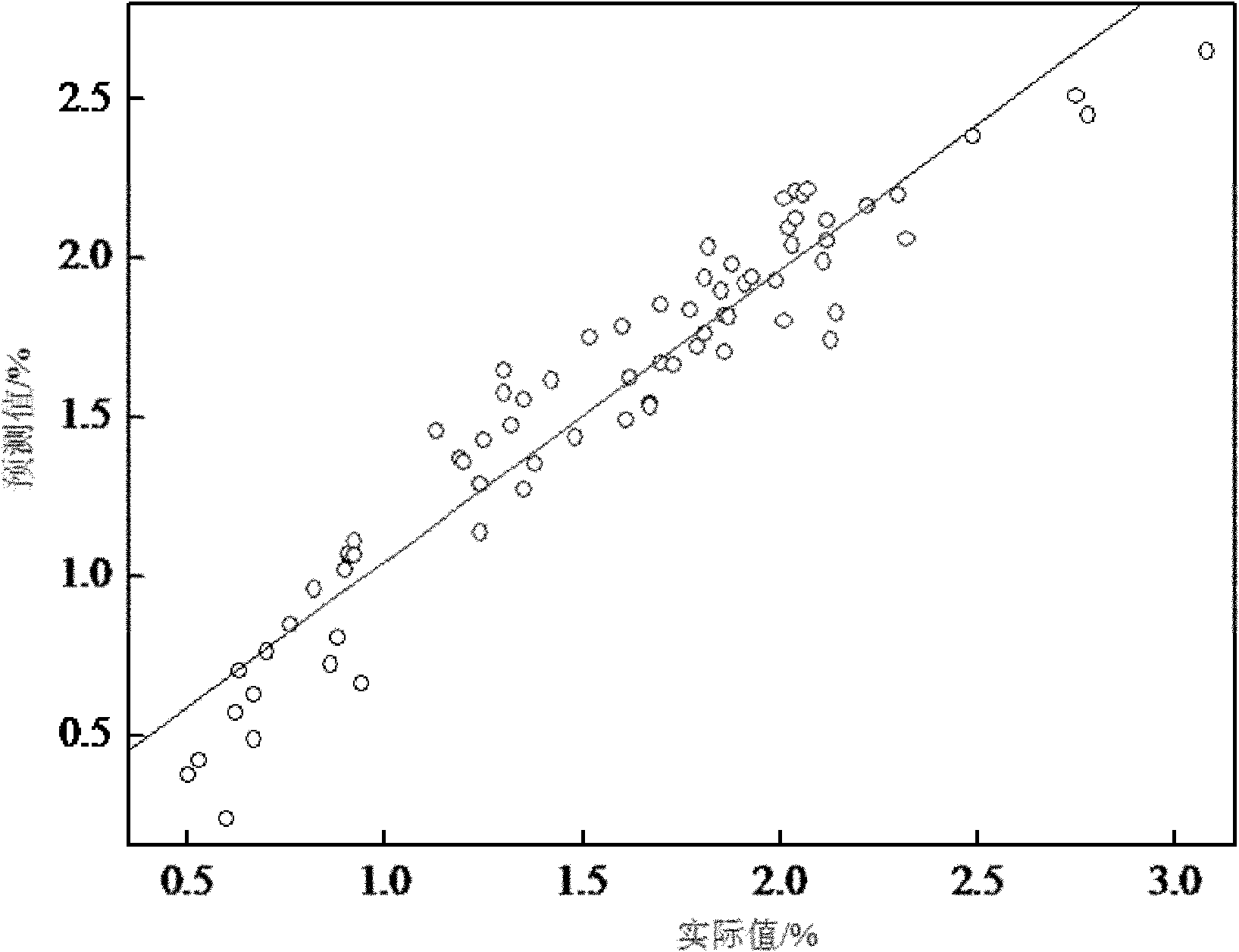
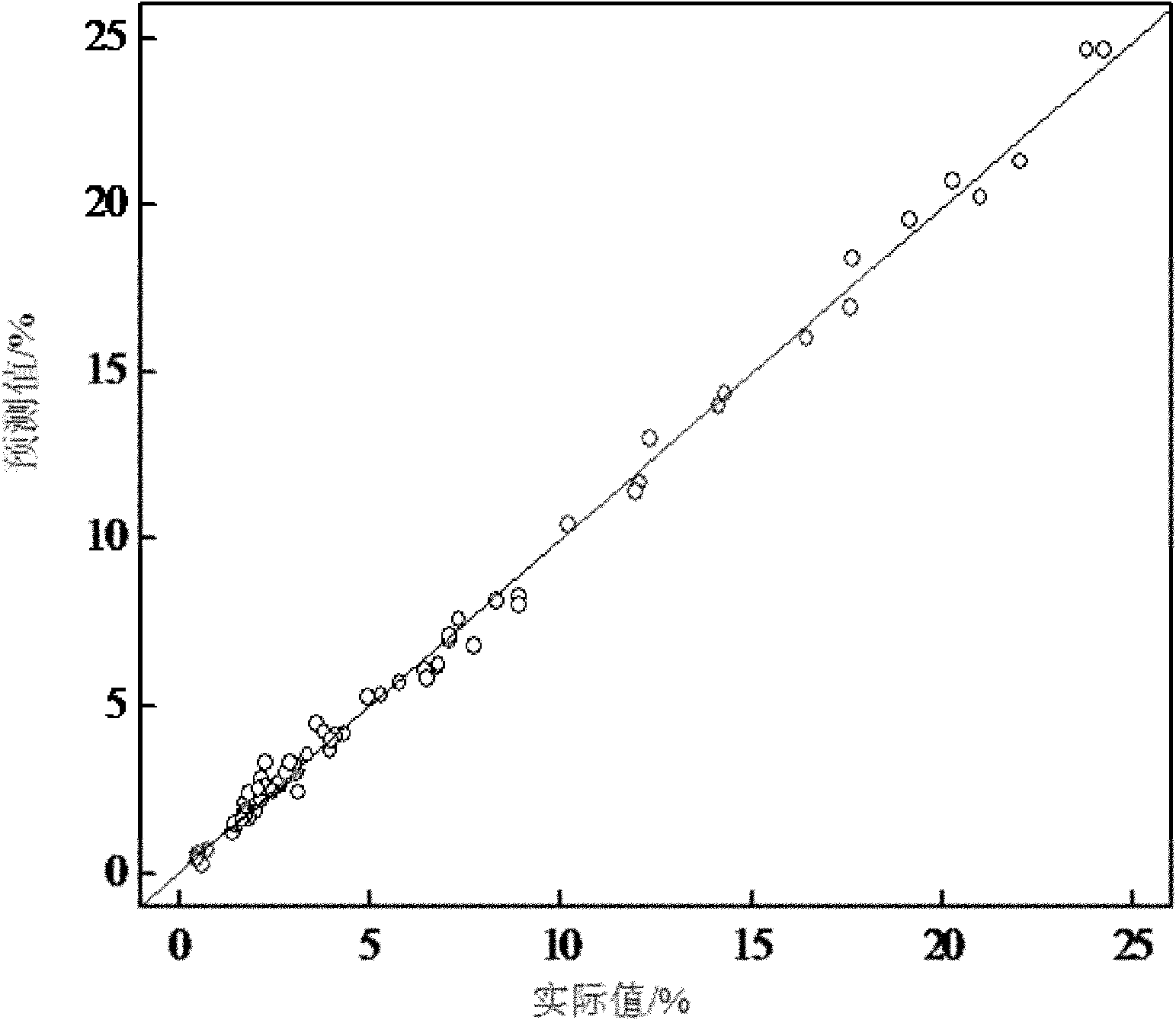
[0009] Specific embodiment one: the rapid analysis method in the emamectin benzoate reaction process of this embodiment is as follows: The abamectin B1a concentration is the sample of 0~25% and the sample of double protective substance concentration is 0.5%~3.5%, set up the sample database; -1 ~4000cm -1 Collect sample spectra within a certain range and establish a sample spectral library; 3. Use the method of combining the average value and normal distribution to select samples with different contents and different reaction times; 4. Use the derivative method and normalization method to predict the selected sample spectra. Treatment; 5. Utilize the methyl and methylene, hydroxyl and amino groups in Abamectin B1a at a wavelength of 8800-4200cm -1 The information of combined frequency and double frequency absorption peaks between these information is used to establish a standard sample model; 6. The partial least squares method is used to establish a quantitative analysis mode...
specific Embodiment approach 2
[0028] Embodiment 2: The difference between this embodiment and Embodiment 1 is that in the process of optimizing the analysis model in step 6, the spectrum interval and the number of principal components are determined according to the model evaluation index determination coefficient and the corrected mean square error. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0029] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is step 2: using a Fourier transform near-infrared spectrum analyzer at a wavelength of 12000cm -1 ~4000cm -1 Collect the sample spectrum within the range, build a sample spectrum library, and the resolution of the Fourier transform near-infrared spectrum analyzer is 8cm -1 , the number of scans is 32 times; step 3: select samples with different contents and different reaction times by means of combining the average value and normal distribution; step 4: use the derivative method and the normalization method to preprocess the selected sample spectrum; Step 5: Use Abamectin B1a methyl group and methylene group, hydroxyl group and amino group at a wavelength of 8800-4200cm -1 The information of the combined frequency and double frequency absorption peaks between these information establishes the standard sample model; Step 6: adopts the partial least squares method to est...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com