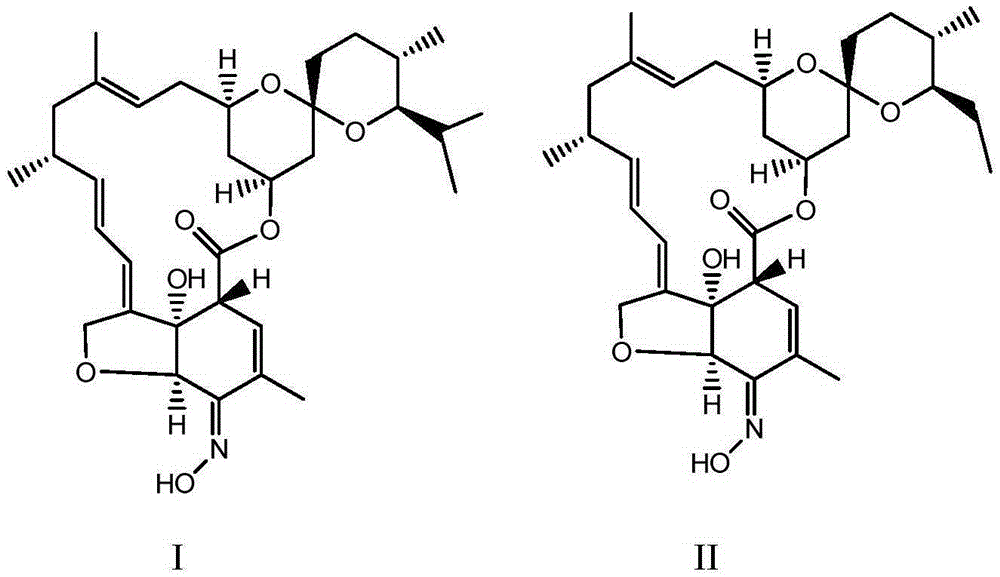
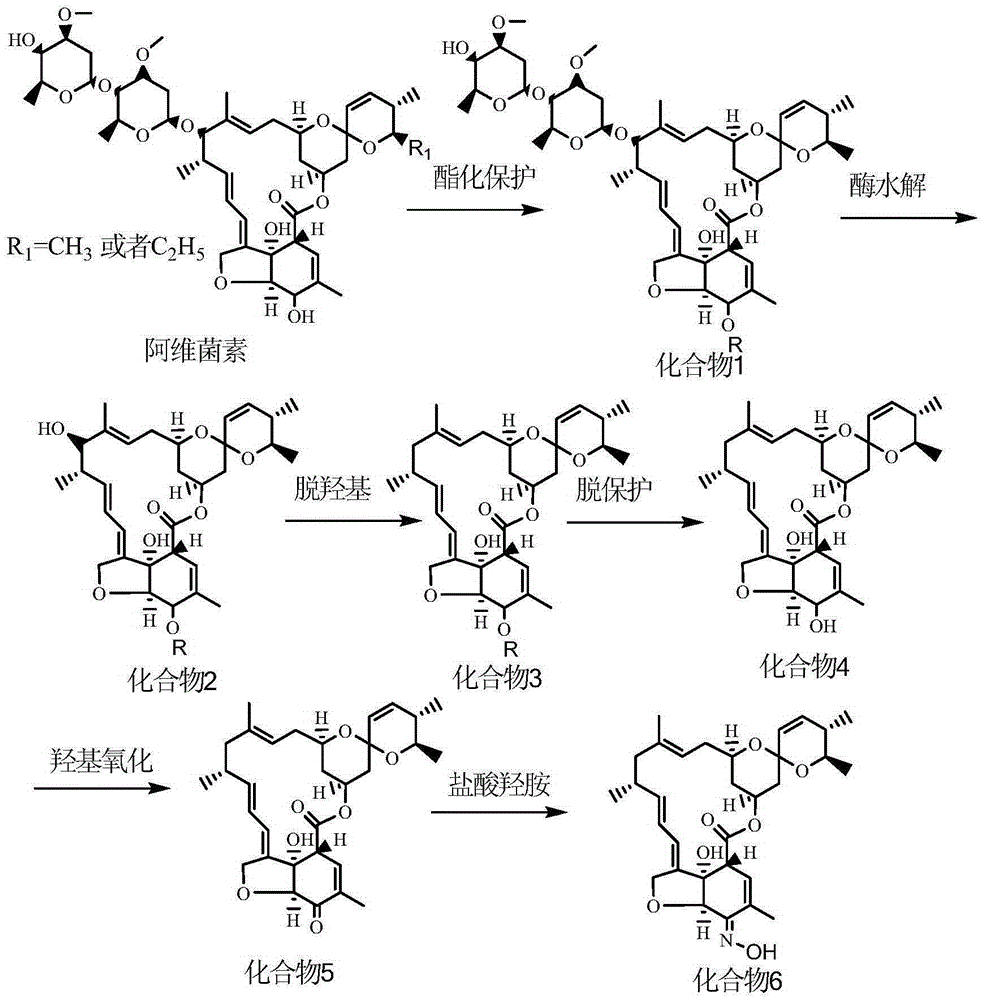
Method for synthesizing milbemycin oxime compound
A technology of milbe oxime and compounds, which is applied in the field of preparation of milbe oxime compounds, can solve problems such as research and development limitations, and achieve the effects of mild reaction conditions, high yield, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A: hydroxyl protection reaction: take 50g abamectin raw material and dissolve in 500ml dichloromethane. Turn on the reactor to stir, set the reactor to be kept at 10°C, and stir. First add 13.2g of triethylamine, then add 8.8g of acetic anhydride, after 3 hours of reaction, add 200ml of water to wash after the reaction, adjust the pH of the water phase to 9.5, stir and wash, then stand to separate the phases, and use dichloromethane for the water phase Extract twice, combine the organic phase, wash the organic phase with saturated NaCl, separate the phases, dehydrate the organic phase with anhydrous sodium sulfate, filter, concentrate, crystallize, filter and dry to obtain 52.8 g of compound 1.
[0039] B: Hydrolysis reaction: Add compound 1 in step A to the reactor, add 5u glycoside hydrolase, stir and hydrolyze at 40°C for 5 hours, extract and dry to obtain 33.8 g of compound 2.
[0040] C: Hydroxyl removal reaction: Dissolve the compound 2 in step B in 200ml tetrahydr...
Embodiment 2
[0045] A: hydroxyl protection reaction: take 50g abamectin raw material and dissolve in 200ml chloroform. Turn on the reactor to stir, set the reactor to be kept at 30°C, and stir. First add 7.1g of diethylamine, then add 29.2g of benzyloxycarbonyl chloride, react for 6 hours, add 200ml of water to wash after the reaction, adjust the pH of the water phase to 9.5, stir and wash, let stand to separate the phases, and wash the water with three Methyl chloride was extracted twice, and the organic phase was combined. The organic phase was washed with saturated NaCl. After phase separation, the organic phase was dehydrated with anhydrous sodium sulfate, filtered, concentrated, crystallized, and filtered to dry to obtain 51.4 g of compound 1.
[0046] B: Hydrolysis reaction: Add compound 1 in step A to the reactor, add 20u glycoside hydrolase, stir and hydrolyze at 50°C for 3 hours, extract and dry to obtain 34.1 g of compound 2.
[0047] C: Hydroxyl removal reaction: Dissolve compo...
Embodiment 3
[0052] A: hydroxyl protection reaction: take 50g abamectin raw material and dissolve in 100ml ethyl acetate. Turn on the reactor to stir, set the reactor to be kept at 50°C, and stir. First add 14.2g of pyridine, then add 22g of di-tert-butyl dicarbonate, react for 8 hours, add 200ml of water to wash after the reaction, adjust the pH of the water phase to 9.5, stir and wash, let stand to separate the phases, and wash the water with ethyl acetate The ester was extracted twice, the organic phase was combined, and the organic phase was washed with saturated NaCl. After phase separation, the organic phase was dehydrated with anhydrous sodium sulfate, filtered, concentrated, crystallized, filtered and dried to obtain 50.5 g of compound 1.
[0053] B: Hydrolysis reaction: Add compound 1 in step A to the reactor, add 50 u of glycoside hydrolase, stir and hydrolyze at 30°C for 6 hours, extract and dry to obtain 32.1 g of compound 2.
[0054] C: Hydroxyl removal reaction: Dissolve the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com