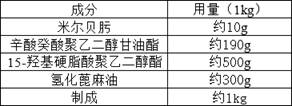
Patents
Literature
36 results about "Milbemycin oxime" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Milbemycin oxime (trade name Interceptor, marketed by Elanco) is a veterinary drug from the group of milbemycins, used as a broad spectrum antiparasitic. It is active against worms (anthelmintic) and mites (miticide).
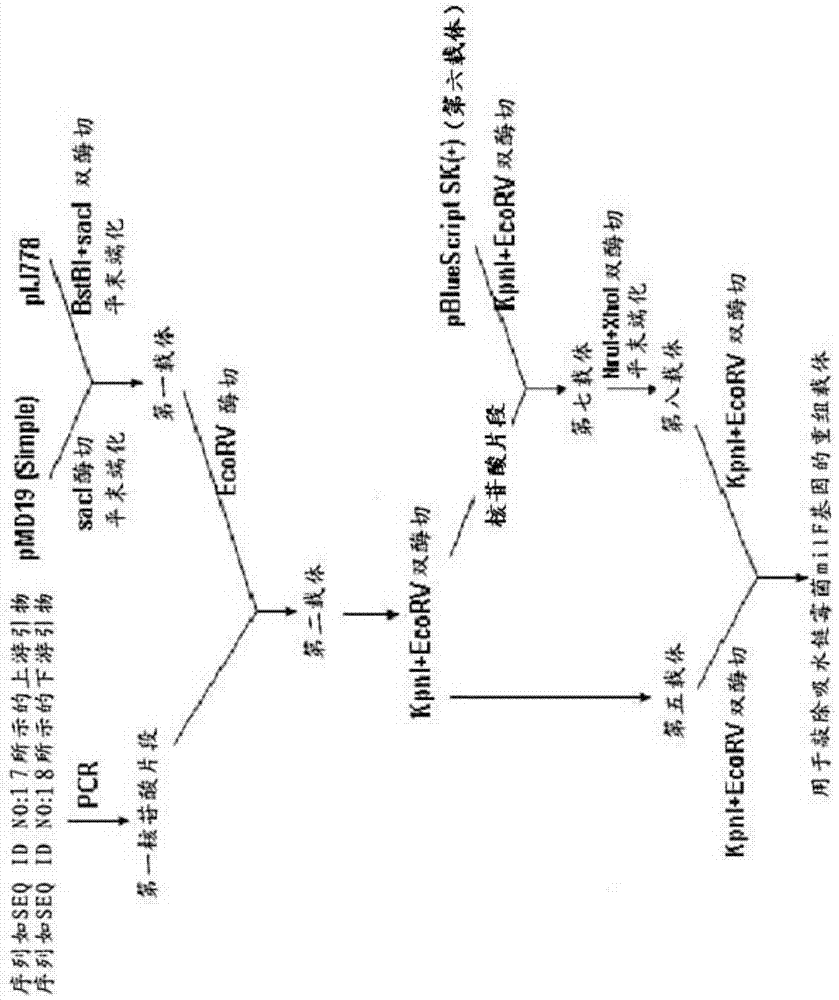
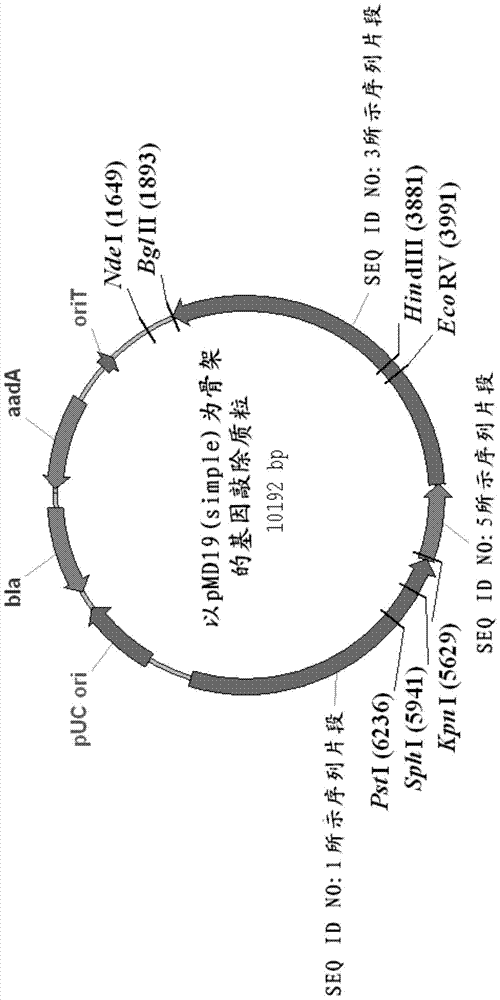
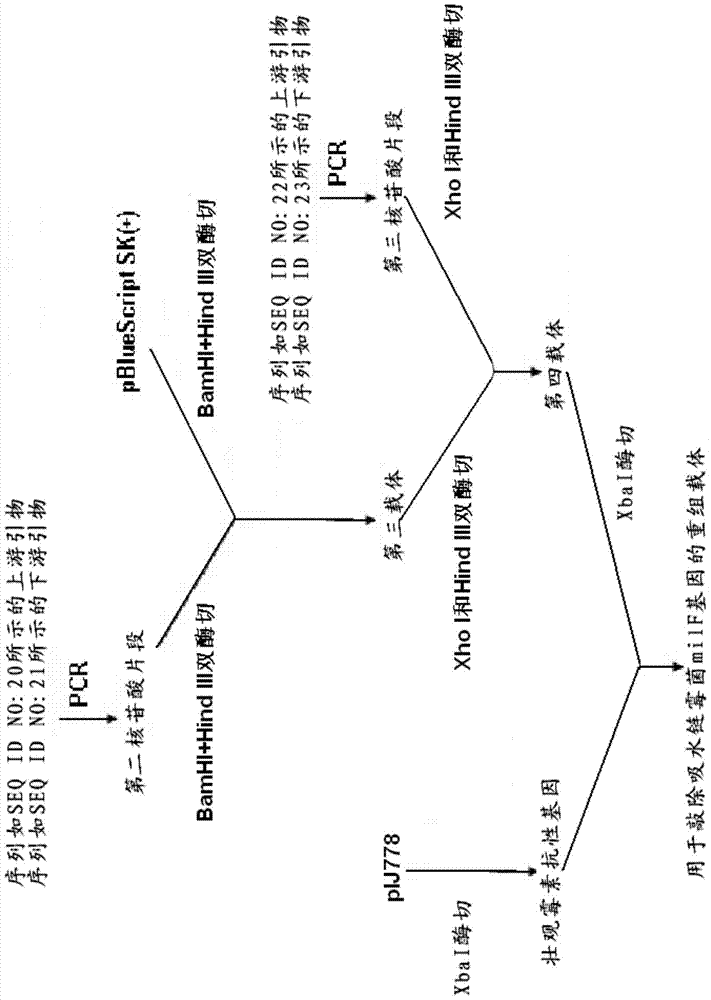
Streptomycete generating 5-keto-milbemycins and method for producing 5-keto-milbemycins
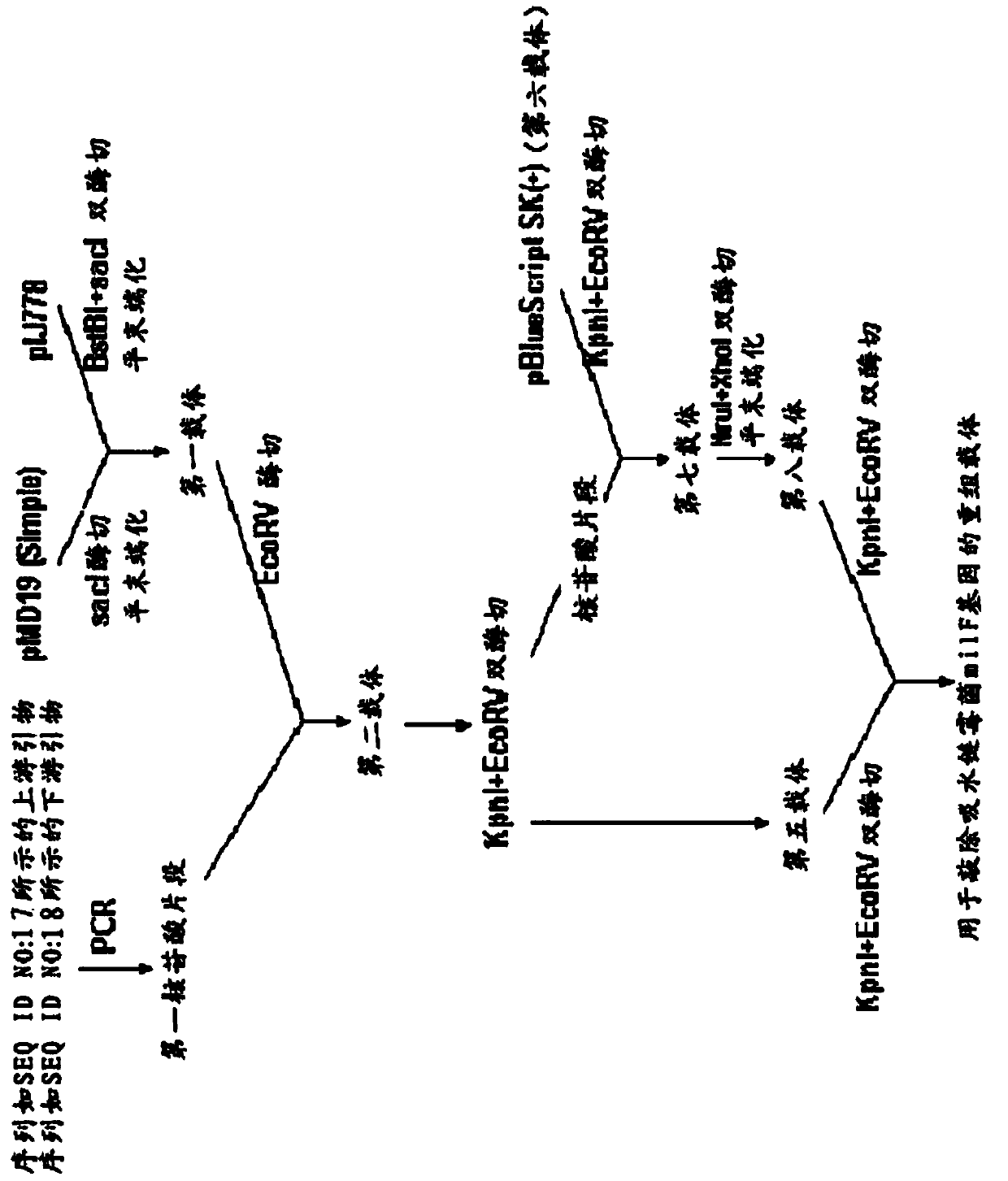
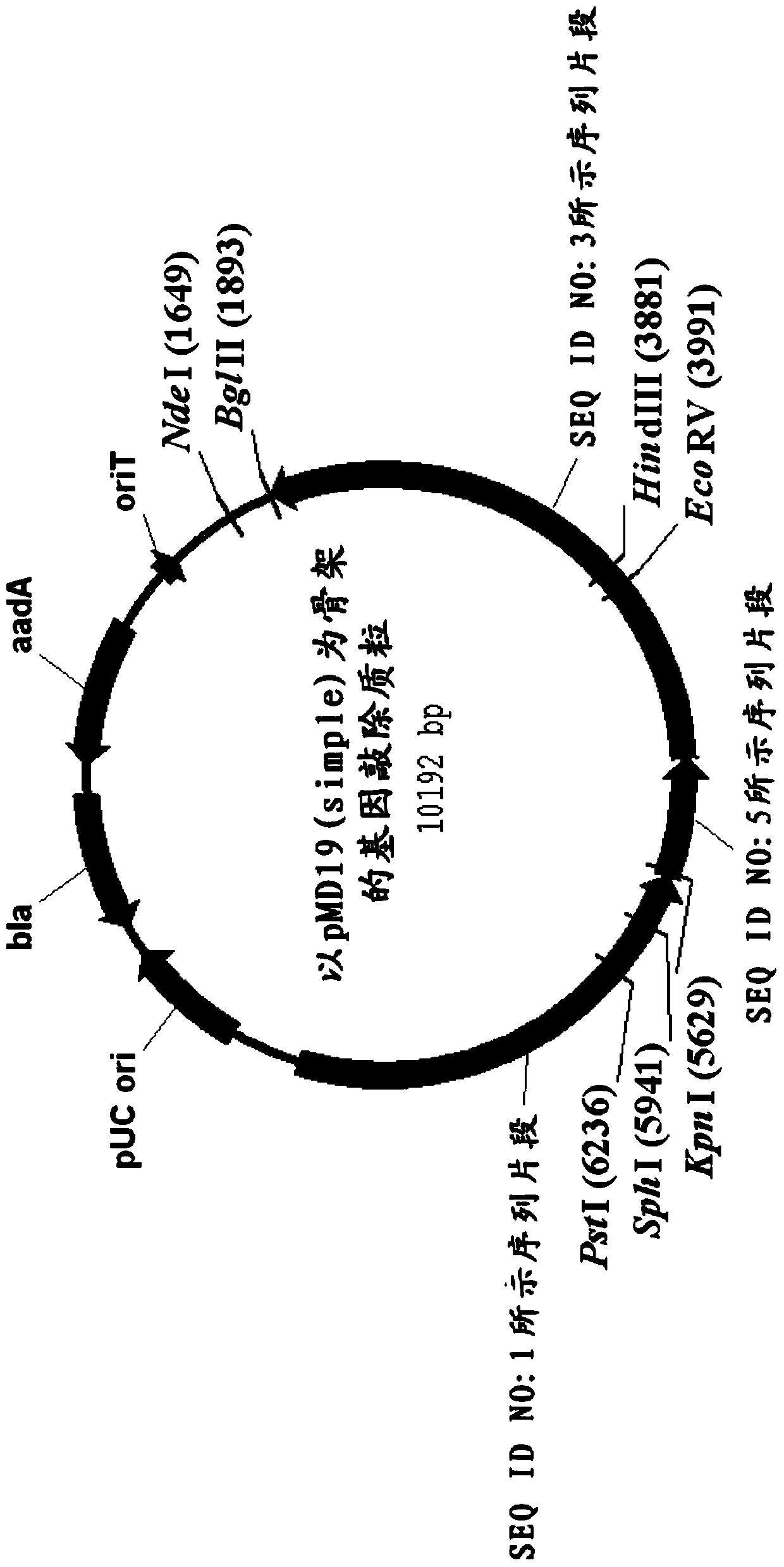
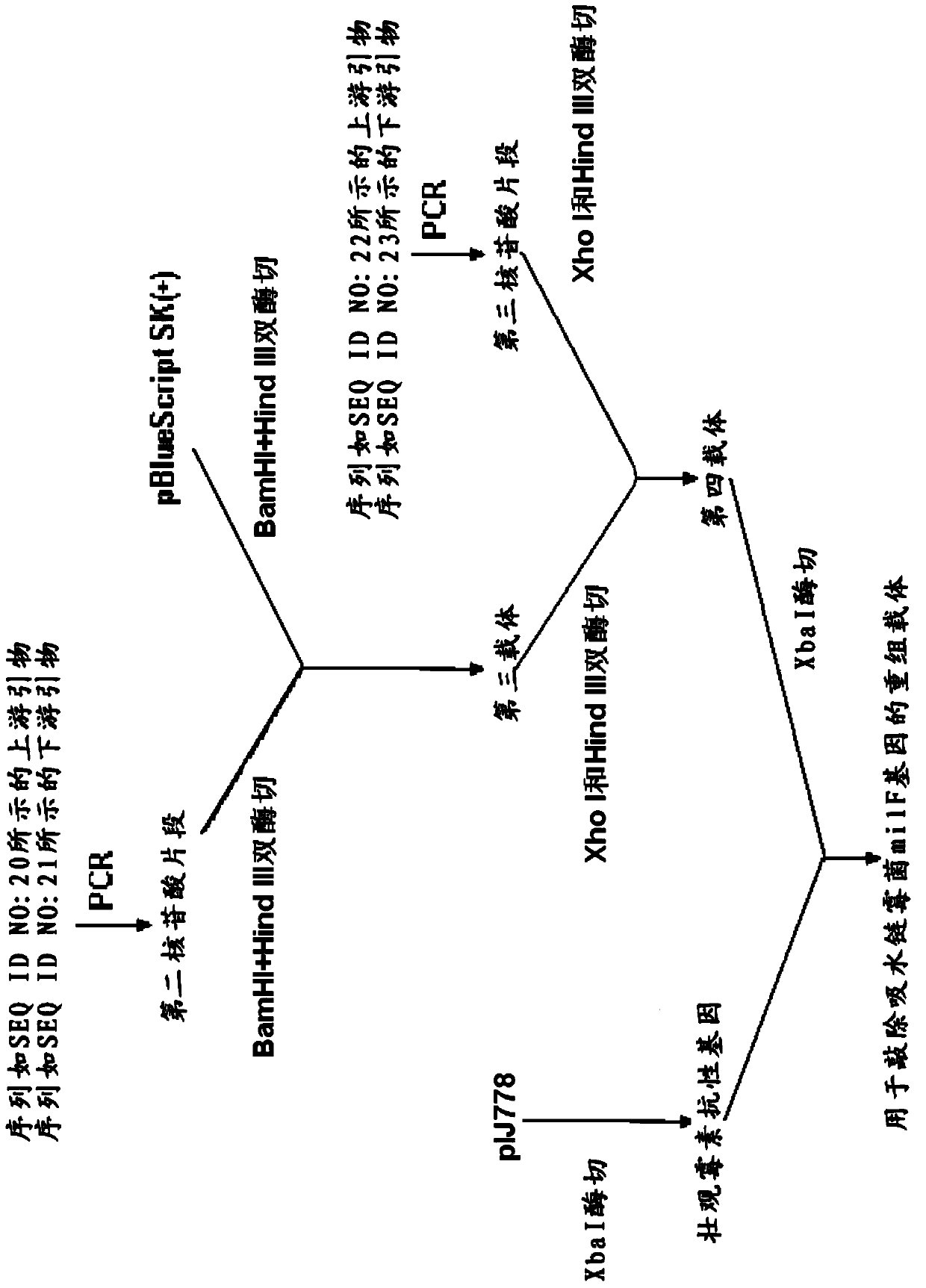
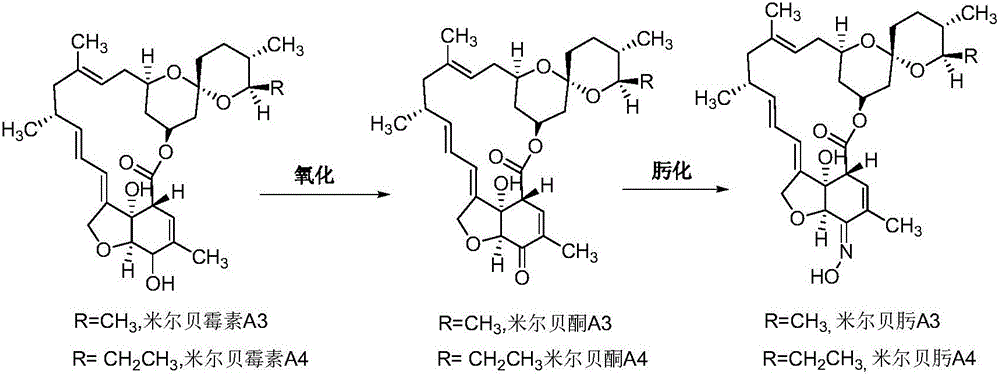
The invention relates to the technical field of gene recombination, and particularly relates to streptomycete generating 5-keto-milbemycins and a method for producing 5-keto-milbemycins. The invention provides a recombinant vector for knocking out the milF gene of streptomycete, including the homologous fragment of the milF gene, wherein the sequence of the fragment is obtained by losing 105-767 nucleotides from the nucleotides from the sites 158-924 in the nucleotide sequence shown by SEQ ID No.2. The invention also provides an establishment method of the recombinant vector, a method for knocking out the milF gene in streptomycete by use of the recombinant vector and streptomycete without milF gene. The streptomycete can be directly fermented to generate 5-keto-milbemycins, so that the synthesis process of milbemycin oxime is simplified, and the pollution caused by the traditional chemical synthesis method is avoided.
Owner:ZHEJIANG HISUN PHARMA CO LTD
A method of preparing high-purity milbemycin oxime
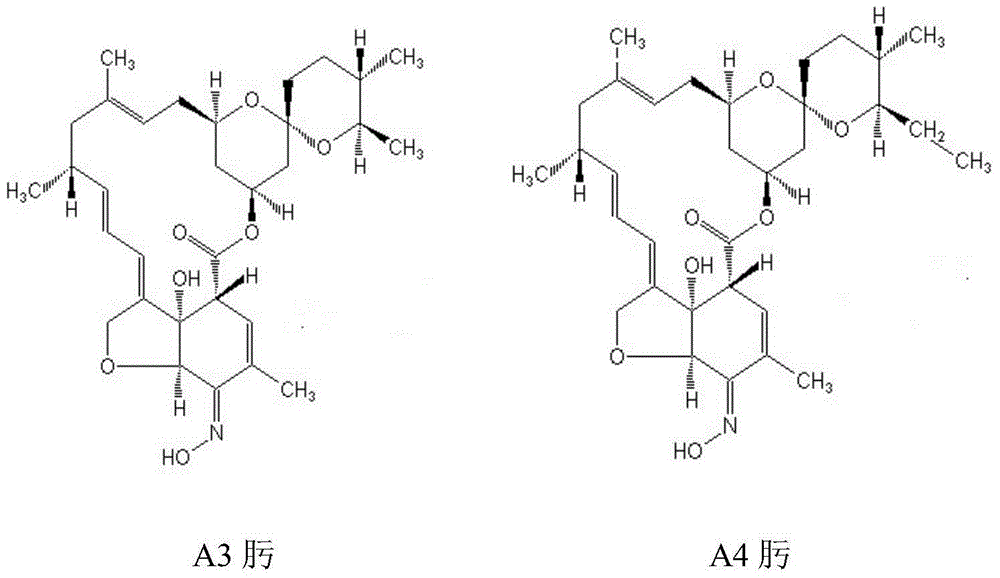
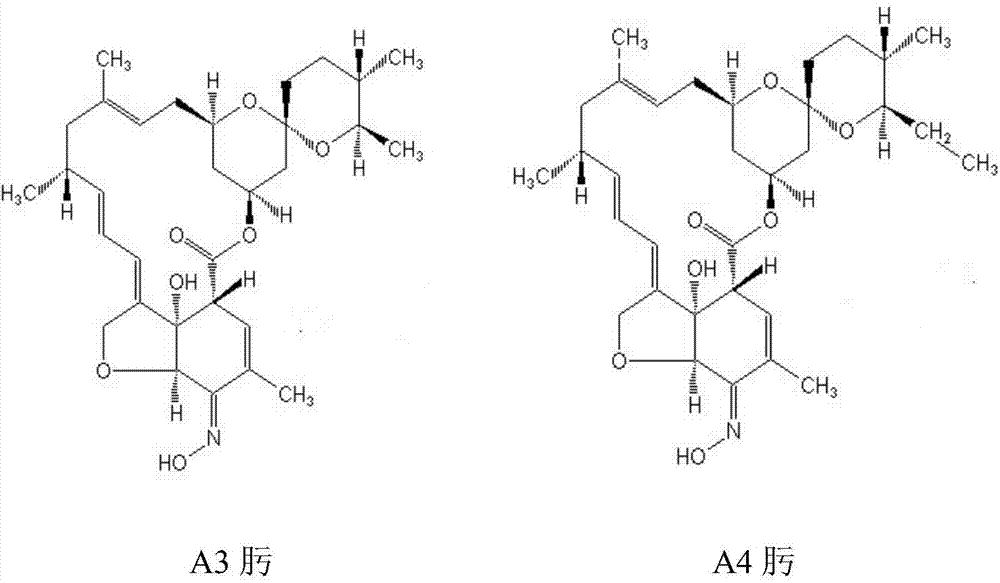
A method of preparing high-purity milbemycin oxime is disclosed. The method includes extracting milbemycin A3 and milbemycin A4 from a fermentation liquor, performing preliminary purification, oxidizing with an oxidant low in oxidizing activity, performing silica gel chromatography to increase the content of milbemycin A3 ketone and milbemycin A4 ketone to a value higher than 75% from a value lower than 25%, naturally crystallizing during concentration to increase the content of the milbemycin A3 ketone and the milbemycin A4 ketone to a value higher than 95%, performing oximation, and performing resin chromatography to adjust the ratio of the content of milbemycin A3 oxime to the content of milbemycin A4 oxime to be 1:4 and to increase the product purity to be higher than 98%. The method is simple in process, low in equipment requirement, good in universality and suitable for large-scale production.
Owner:CHONGQING DAXIN PHARMA +2
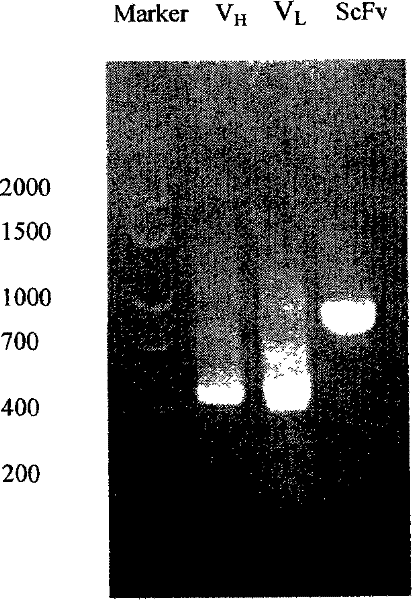
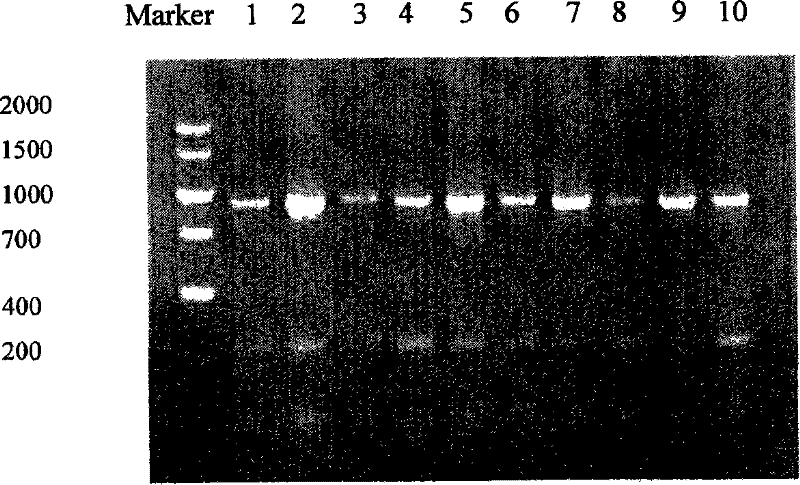
Antibody library of bacteriophages and applications in immunoassay of pesticide residue
InactiveCN101289760AWide range of affinity optionsIncrease screening throughputPeptide librariesMicroorganism librariesSingle-Chain AntibodiesPesticide residue
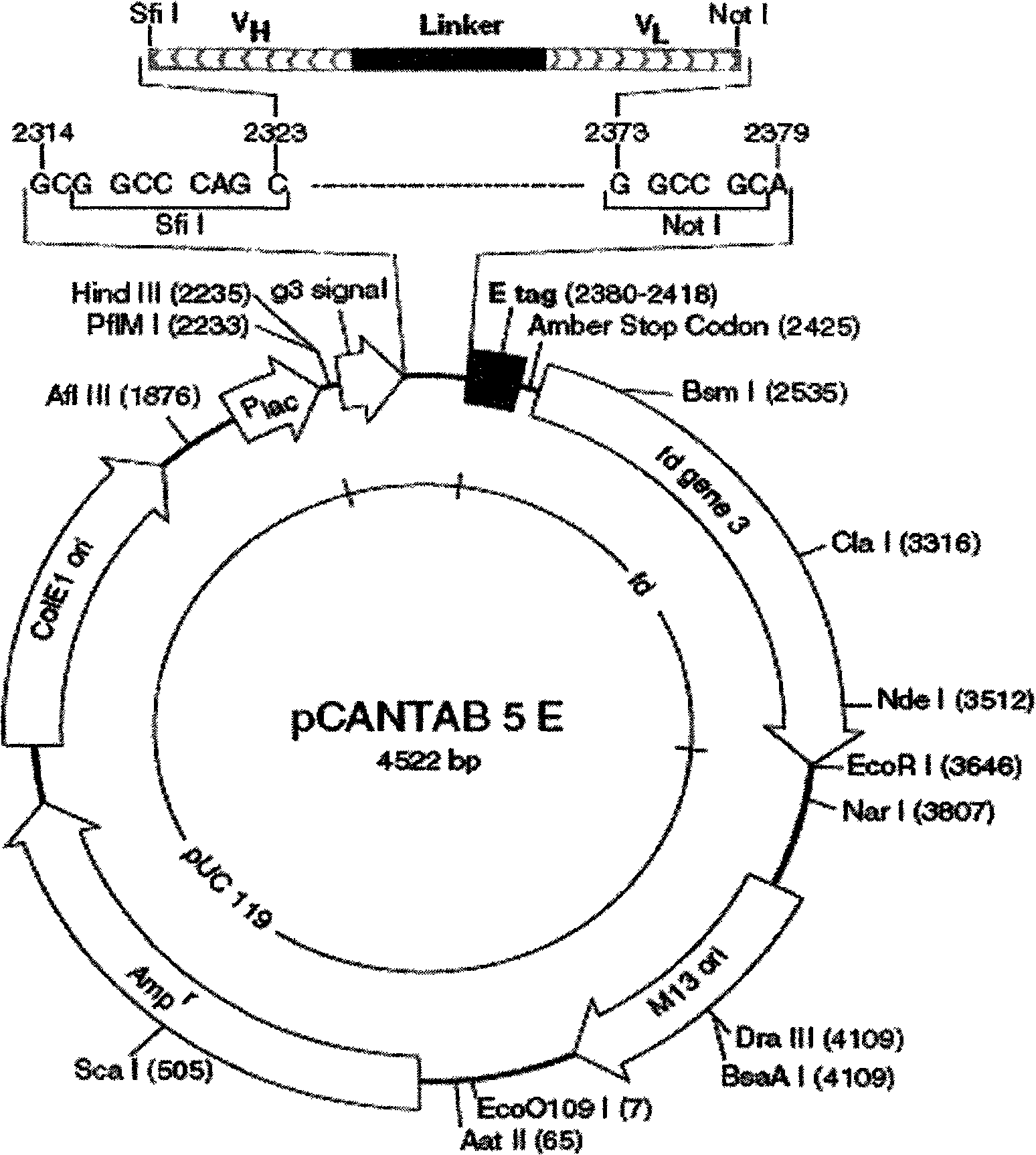
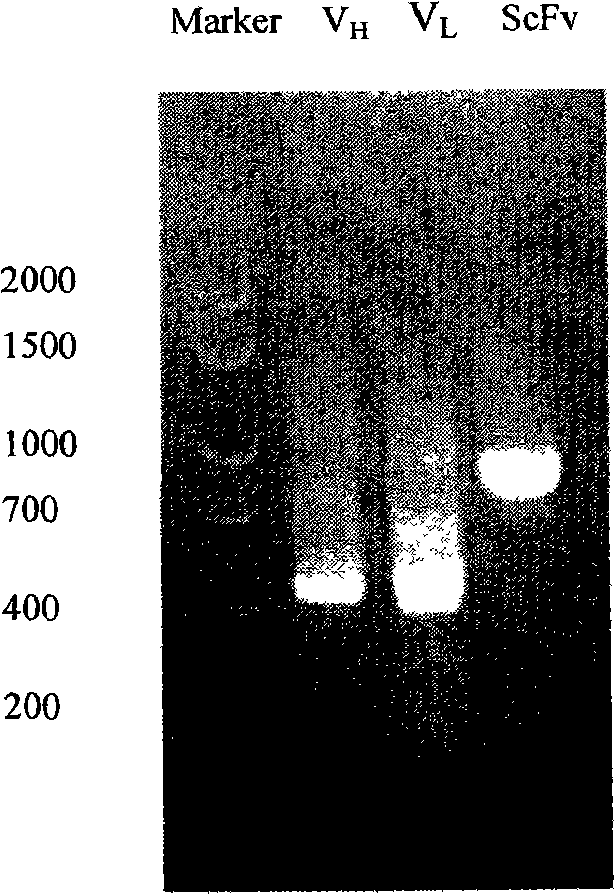
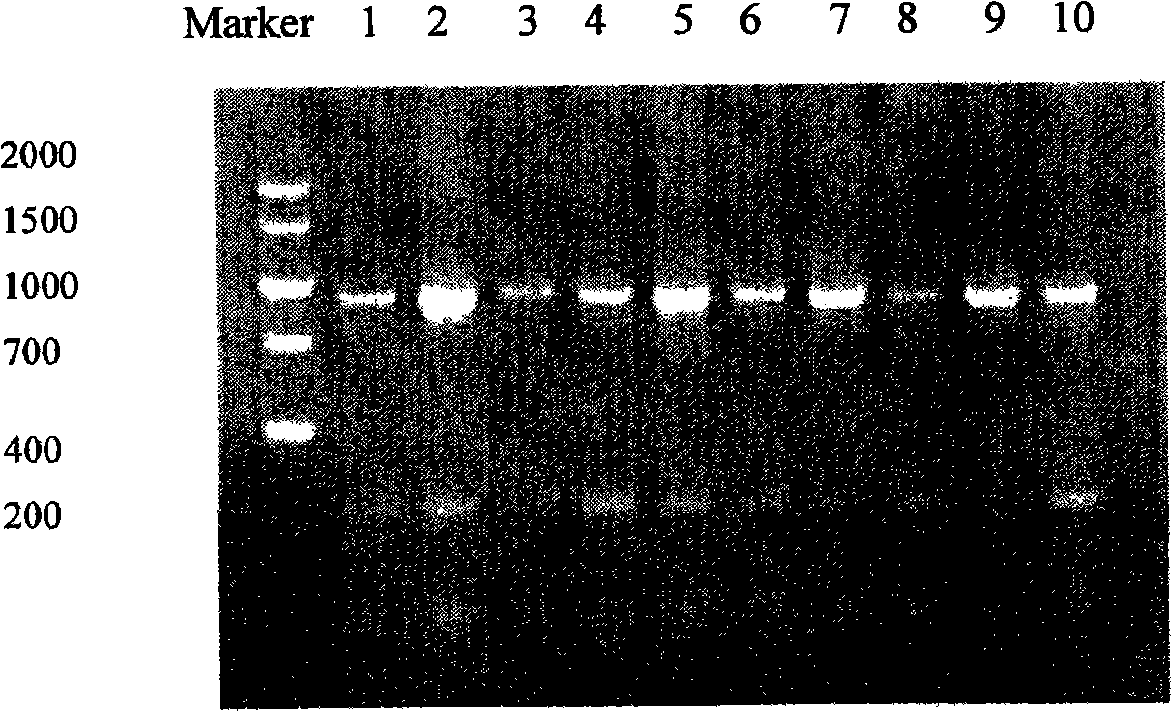
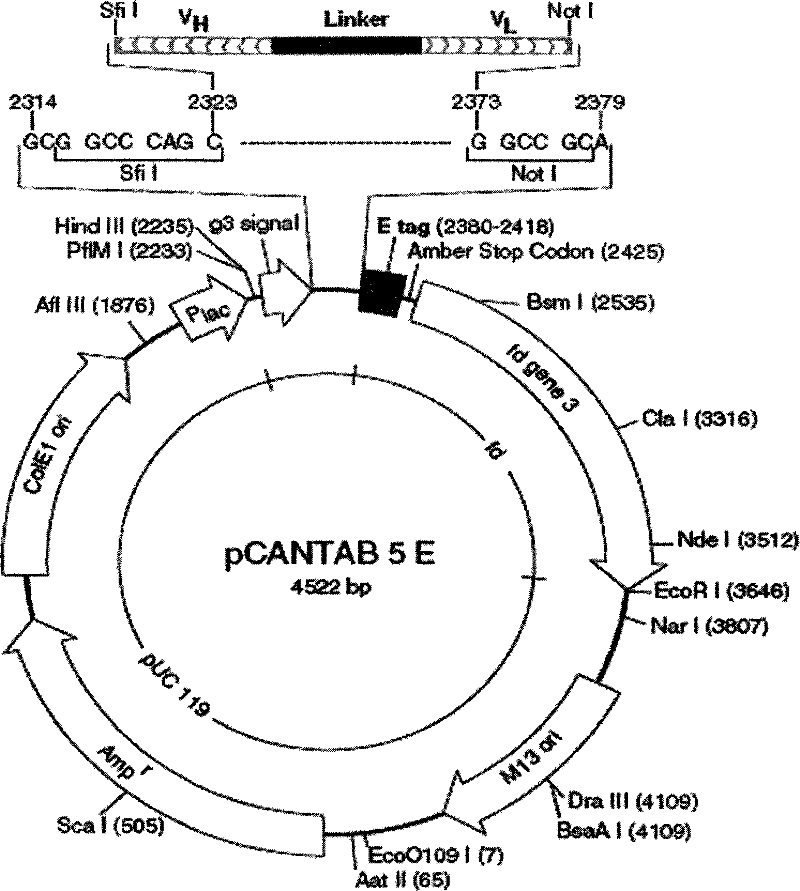
The invention relates to a phage antibody library which assembles an ScFv gene fragment between Restriction Enzyme cutting sites of SfiI and NotI of a pCANTAB5E carrier. The phage antibody library is characterized in that the ScFv gene fragment can be affinitive and enriched with antigens of 16-membered macrolide agricultural chemicals to form a soluble single-chain antibody and the phage antibody library is applied to immunoassay of pesticide residue. The phage antibody library has the advantages that the antibody with high affinity can be obtained without animal immunization, the test period is short, the antibody library is the phage antibody library which takes small molecular milbemycin oxime of a 16-membered macrolide generic structure as immunogen to construct, the antibody library theoretically can directly obtain a specific antibody library of 16-membered macrolide compound through screening, the screening flux is high, the efficiency is high, the specificity is strong, and the affinity selecting range is wide, so that the phage antibody library has wide application prospect in the aspects such as agricultural chemical antibody preparation, testing technique development and the like.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Preparation method of milbemycin oxime
ActiveCN105254644AIncrease production capacityLow costOrganic chemistryHydroxylamine HydrochlorideKetone
The invention provides a preparation method of milbemycin oxime. The preparation method of milbemycin oxime comprises the following steps: extracting milbemycins, namely taking milbe mycelia obtained through fermentation as a raw material, extracting, concentrating, extracting, concentrating, extracting again and concentrating again, so as to obtain milbemycin; preparing milbemycin ketone, namely taking the milbemycin as a raw material, establishing an oxidation reaction system, after reaction is finished, filtering, concentrating, extracting and concentrating the reaction system, so as to obtain an intermediate product milbemycin ketone; synthesizing milbemycin oxime, namely dissolving milbemycin ketone with methanol and dioxane, dropwise adding hydroxylamine hydrochloride solution and reacting, concentrating the reaction system, extracting, drying and concentrating, so as to obtain a milbemycin oxime crude product; and purifying milbemycin oxime, namely crystallizing the milbemycin oxime crude product with a mixed solvent of trichloromethane and normal heptane, dissolving a crystalline product with ethanol, dropwise adding into water while stirring for carrying out crystallization, filtering, and drying, so that the milbemycin oxime finished product is obtained. The preparation method of the milbemycin oxime has the advantages that productivity is greatly improved, and cost is obviously reduced, so that the preparation method provided by the invention is obviously better than an existing preparation method.
Owner:HUBEI HONCH PHARMA
Milbemycin oxime compound and preparation method thereof
InactiveCN103896961AHigh yieldSimple and fast operationOrganic chemistryNMR - Nuclear magnetic resonanceAvermectin B1a
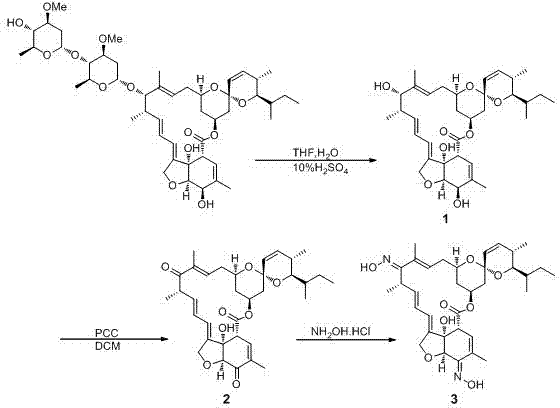
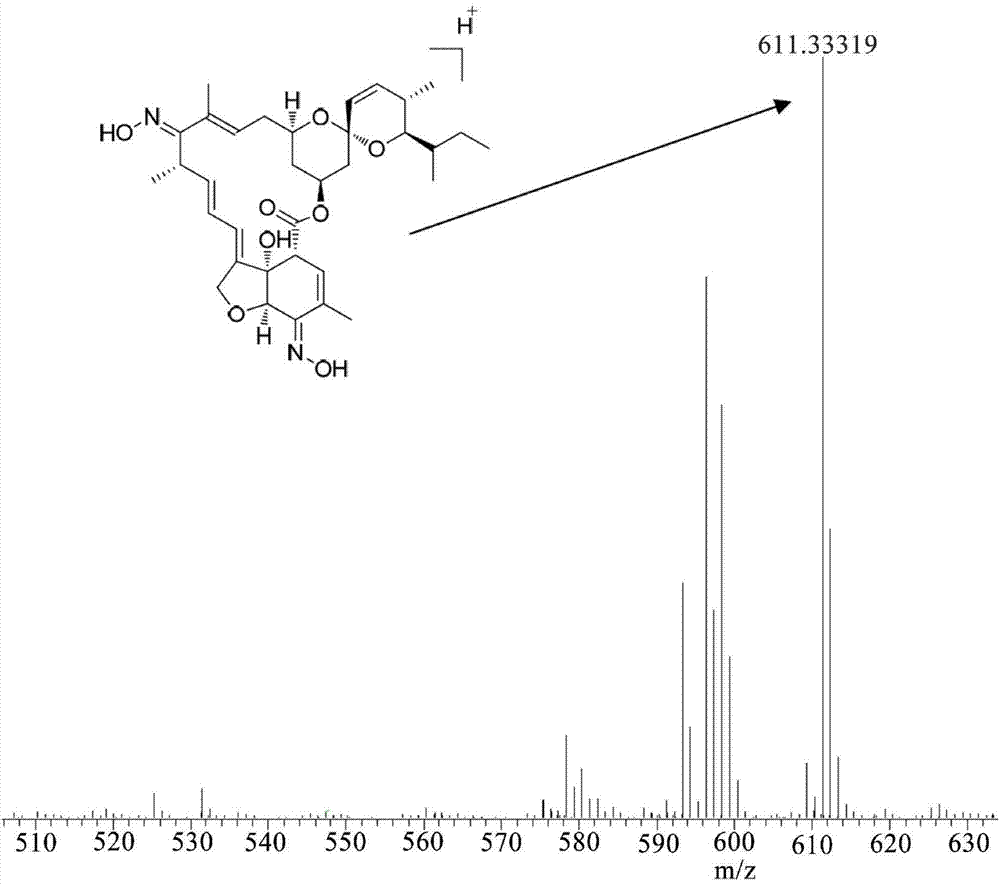
The invention discloses a milbemycin oxime compound and a preparation method thereof. The compound as shown in a general formula (I) in the specification is prepared by the steps of carrying out hydrolysis reaction on avermectin B1a to remove C13-position glycosyl, oxidizing both C5-position hydroxyl and C13-position hydroxyl into keto through oxidation reaction, and finally converting the two kinds of keto into oximido, wherein the structure of the compound is confirmed by virtue of the mass spectrum and nuclear magnetic resonance analysis testing technology. According to the milbemycin oxime compound and the preparation method, the milbemycin oxime compound is prepared by starting materials which are cheap and easily obtained; the preparation method has the characteristics of mild reaction condition, simpleness in operation process, low cost and higher yield.
Owner:WUHAN UNIV
Synthetic method for milbemycin oxime
ActiveCN105061457AImprove conversion rateMild conditionsOrganic chemistryKetoneHydroxylamine Hydrochloride
The invention provides a synthetic method for milbemycin oxime. The method comprises the following steps that 1, an oxidation reaction is performed, wherein milbemycins is used as raw materials, pypocholoride or chlorite is used as an oxidizing agent, piperidines free radical of nitroxide is used as a catalyst, a halide is used as a promoter, all the materials are subjected to the reaction in a dichloromethane solvent at the temperature being minus 5-15 DEG C for 0.5-4 h, and postprocessing is performed on a reaction product to obtain an intermediate product of milbemycin ketone; 2, an oximation reaction is performed, wherein methyl alcohol and 1,4-dioxane are used as a reactive solvent, hydroxylamine hydrochloride is used as an oximation reagent, the reaction is performed at the temperature being 25-35 DEG C for 10-16 h, and postprocessing and purification are performed to obtain milbemycin oxime. According to the method, industrial production of the milbemycin oxime is achieved in China for the first time, and the yield of the obtained product is higher than that of similar products both at home and abroad. Due to the fact that the pypocholoride is used as the oxidizing agent, pyridine nitroxide free radical is used as a catalyst, conditions are mild, few side reactions exist, the yield is high, and the cost is low.
Owner:HUBEI HONCH PHARMA
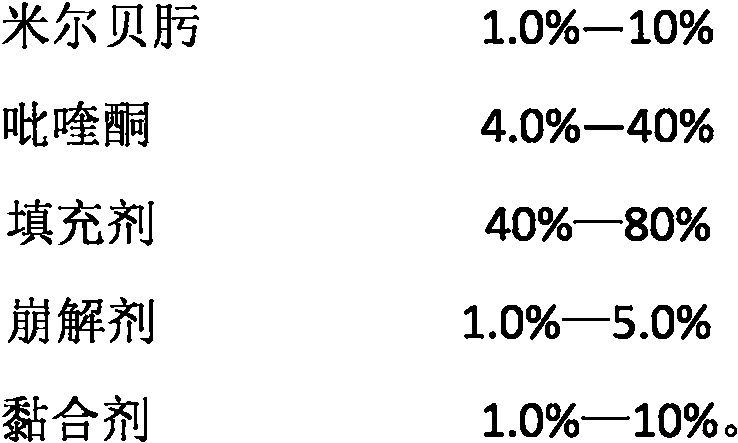
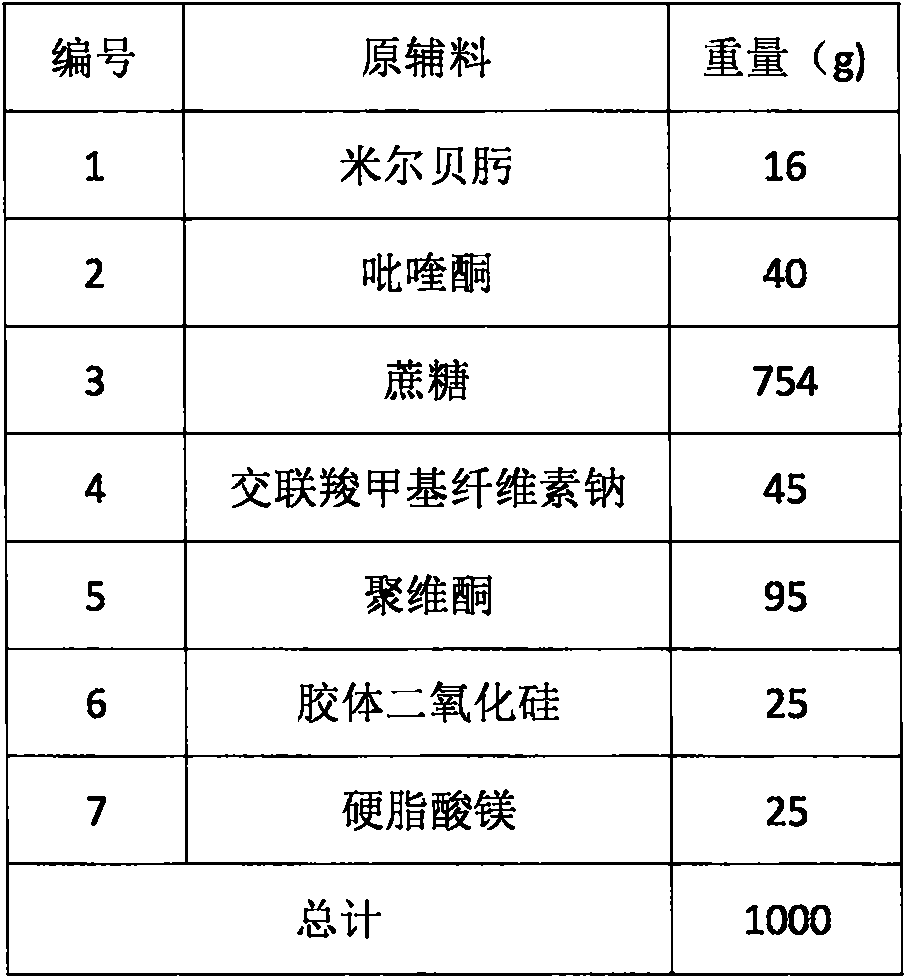
Compound preparation for expelling in-vivo and in-vitro parasites from dogs and cats and preparation method thereof
The invention relates to a compound preparation for expelling in-vivo and in-vitro parasites from dogs and cats and a preparation method thereof. The compound preparation mainly comprises milbemycin oxime and praziquantel, wherein the milbemycin oxime is a novel and specific 16-membered ring macrolide medicament for preventing and controlling the in-vivo and in-vitro parasites of the dogs and the cats, which can specially prevent and control heartworms and efficiently prevent and control in-vivo parasites such as hookworms, roundworms, whipworms, belly worms and the like and in-vitro parasites such as hair follicle mites, scabies, louses, fleas and the like; and the praziquantel is effective to nematodes, trematodes and tapeworms in animal bodies. The milbemycin oxime and the praziquantel expel the parasites complementarily. The two medicaments are combined, so that a parasite expelling range is expanded and one-step parasite expelling effect is improved. The invention also provides a method for preparing compound tablets. The tablets prepared by the method can be released rapidly and dispersed fully.
Owner:TIANJIN RINGPU BIO TECH
Milbemycin oxime separation and purification method
The invention provides a milbemycin oxime separation and purification method. The milbemycin oxime separation and purification method comprises the following steps: carrying out crude separation on a sample to be separated through silicagel column chromatography, wherein the silicagel column chromatography adopts wet-process column packing and dry-method sample loading; purifying the product after crude separation by high performance liquid chromatography; concentrating the purified sample by a nanofiltration membrane to obtain concentrated solution; performing reduced-pressure vaporization to the concentrated solution, filtering and drying to obtain a refined product; dissolving the refined product, heating up, dropwise adding isooctyl alcohol, normal heptane or petroleum ether, cooling to be crystallized, and filtering to obtain a crystallized product; dissolving the crystallized product, filtering, dropwise adding filtrate into purified water stirred constantly, after dropwise adding, carrying out suction filtration to obtain a crystal transformation product; drying the crystal transformation product, crushing, and drying again to obtain milbemycin oxime finished product. The method can realize industrial production of milbemycin oxime in China firstly, and the product has high purity and high yield which are higher than those of the similar products at home and abroad.
Owner:HUBEI HONCH PHARMA
Milbemycin oxime and praziquantel flavored tablet and preparation method thereof
PendingCN112220769AIncreased willingness to eat food voluntarilyEasy to administerOrganic active ingredientsInorganic non-active ingredientsPharmaceutical SubstancesOrganic chemistry
The invention relates to a milbemycin oxime and praziquantel flavored tablet and a preparation method thereof. The milbemycin oxime and praziquantel flavored tablet consists of a tablet core and a drug-free coating layer coating the surface of the tablet core, wherein the coating layer can be one or more layers, and the coating layer contains at least one phagostimulant or flavoring agent; and thetablet core consists of pellets, a flow aid and a lubricant. The preparation process of the milbemycin oxime and praziquantel flavored tablet comprises preparation of drug-containing powder, preparation of the pellets, preparation of the tablet core, preparation of the coating layer and preparation of the flavored tablet. The preparation technology is simple, and the main drug and the auxiliary agent can be uniformly mixed together, so that the auxiliary agent can better play a role, the stability of the drug is improved, the taste of the drug is improved, and the milbemycin oxime and praziquantel flavored tablet is suitable for industrial production and has a wide application prospect.
Owner:ZHEJIANG HISUN ANIMAL HEALTH PROD CO LTD
Preparation method of milbemycin oxime intermediate
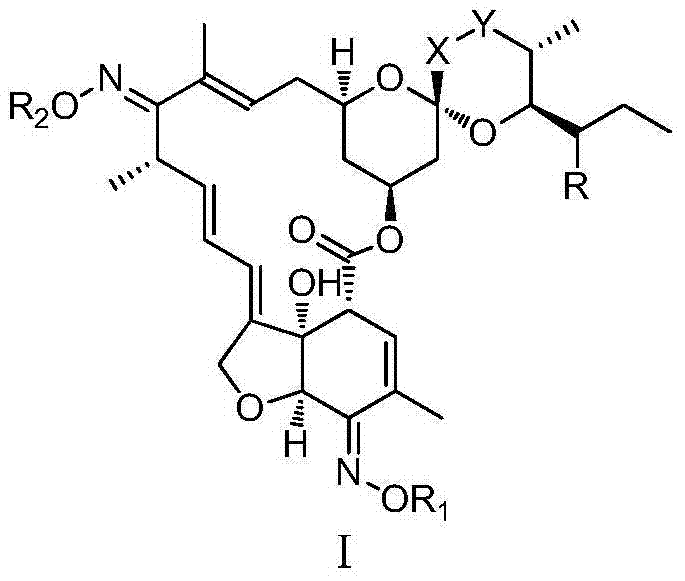
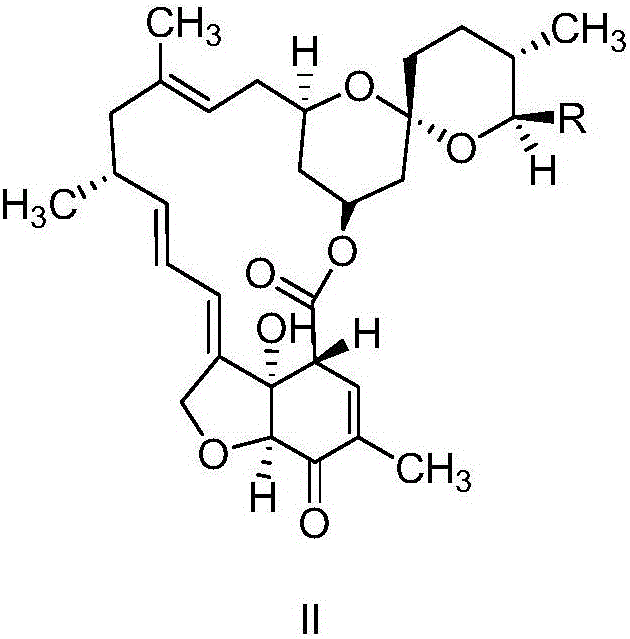
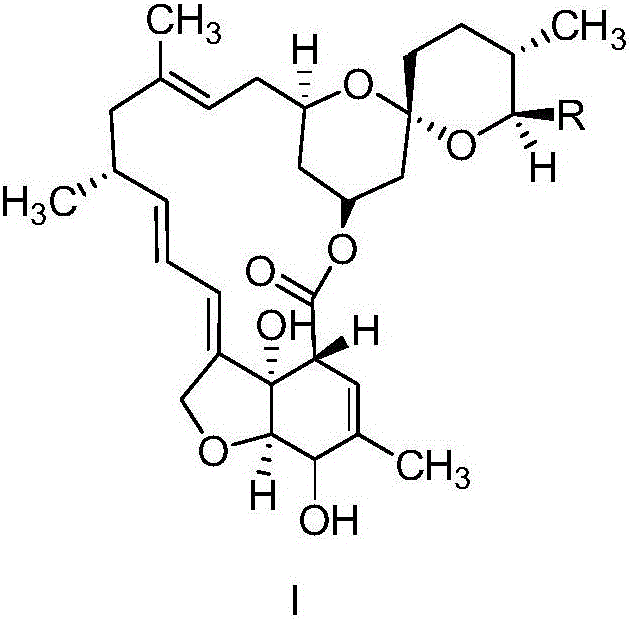
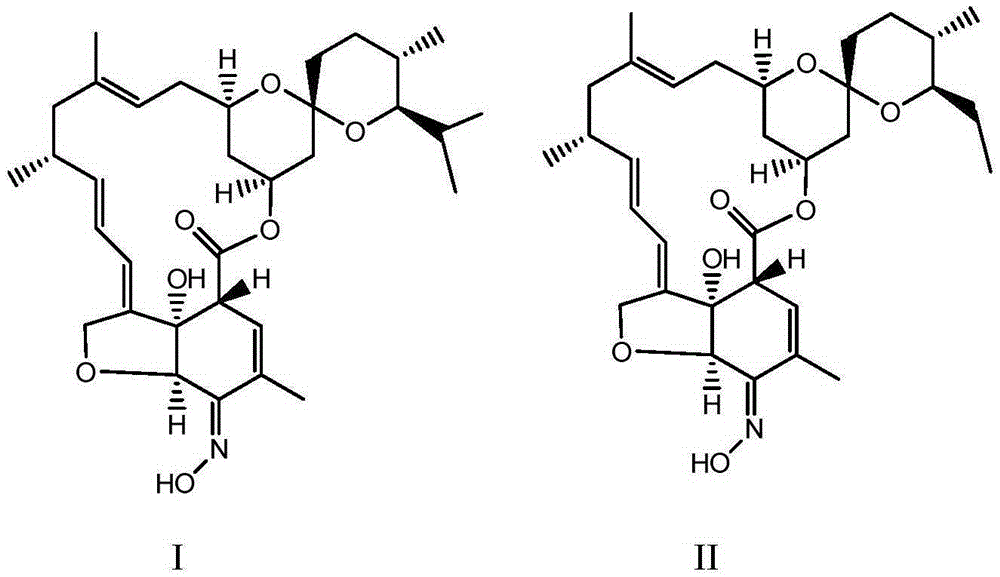
The invention relates to a preparation method of a milbemycin oxime intermediate II. The preparation method comprises the step of reacting milbemycins (I) with pypocholoride as an oxidant in the presence of a catalyst and bromide to generate the milbemycin oxime intermediate II. The preparation method provided by the invention is simple in process operation, high in yield, low in cost, and is very suitable for industrial production. The formula I and the formula II are shown in the description, wherein R is equal to CH3 or C2H5.
Owner:ZHEJIANG HISUN PHARMA CO LTD
LC-MS/MS method for determining milbemycin oxime content of animal plasma
InactiveCN101915818AStrong detection specificityEfficient separationComponent separationColumn temperatureNitrogen gas
The invention discloses an LC-MS / MS method for determining the milbemycin oxime content in animal plasma and belongs to the fields of veterinary drug content determination, pharmacokinetics and residue law. The method comprises the following steps of: adding 4mL of acetonitrile and 0.3g of sodium chloride into 1mL of plasma sample, mixing and then centrifuging the mixture, drying the supernatant by blowing nitrogen gas, adding 3mL of methanol and 5mmol / L of ammonium acetate into the residue in a ratio of 1:9, purifying the mixture by a C18 solid extraction column; and using a liquid chromatography-mass spectrometry instrument for analysis under the following conditions: electrospray ionization, nitrogen gas spray pressure of 49psi, spray voltage of 4800V, nitrogen gas pressure of 20psi, temperature of 300 DEG C, Source CID of 12V; m / z of 536, detection of fragment ions of which m / z is 536 by the full scan, composition of mobile phase by acetonitrile and 0.5mmol / L of ammonium acetate in a ratio of 85:15, flow speed of 0.25mL / min, and column temperature of 20 DEG C. The method has the characteristics of fastness, sensitivity and low detection limit.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Compound milbemycin oxime nanoemulsion anti-parasite medicine and preparation method thereof
InactiveCN102614173AImprove stabilityLow viscosityOrganic active ingredientsAntiparasitic agentsHook wormsNematode
The invention discloses a compound milbemycin oxime nanoemulsion anti-parasite medicine and a preparation method thereof. The diameter of a liquid drop of the nanoemulsion medicine ranges from 1nm to 100nm, the medicine is composed of the following raw materials by mass: milbemycin oxime 0.5%-3.5%, levamisole 0.1%-0.5%, a surface active agent 23.5%-38.5%, oil 3.0%-17.0%, the remaining part is distilled water, and the sum of the mass percentage of the raw materials is 100%. The nanoemulsion preparation is clear and transparent liquid, has good stability and fast absorption, is released to targets, has the advantages of high efficiency, broad spectrum, low toxicity and the like, is a medicine for preventing and treating internal and external parasites of pets, and is mainly used for preventing and treating dirofilariasis and removing internal parasites of nematodes, hookworms, roundworms, whipworms and the like and external parasites of itch mites, nasal mites, hair follicle mites, louses, fleas and the like. The compound milbemycin oxime nanoemulsion anti-parasite medicine solves the problem of water insolubility of milbemycin oxime, is simple in preparation process, high in safety and convenient to popularize.
Owner:NORTHWEST A & F UNIV
A kind of synthetic method of milbexime
ActiveCN105061457BImprove conversion rateMild conditionsOrganic chemistryHypochloriteHydroxylamine Hydrochloride
The present invention provides a method for synthesizing Milbemycin oxime. The method comprises the following steps. (1) Oxidizing reaction: oxidizing Milbemycin, using hypochlorite or chlorite as oxidizers and piperidine nitrogen oxygen free radicals as the catalyst and halide as the catalyst promoter. The oxidation reaction is conducted in a dichloromethane solvent for 0.5-4 hours at −5-15° C. to produce the intermediate product Milbemycin ketone. (2) Oximation reaction: reacting the Milbemycin ketone with a hydroxylamine hydrochloride oximation agent in a 1,4-dioxane reaction solvent for 10-16 hours at 25-35° C. to obtain Milbemycin oxime. The method provided by the present invention realized the industrial production of Milbemycin oxime for the first time domestically. Moreover, the yield of the prepared product is higher than competing products both at home and abroad.
Owner:HUBEI HONCH PHARMA
Oily injection containing antiparasitic agent/polyethylene glycol drug-loading microparticles
InactiveCN103721260AResidue reductionSolution deliveryAntiparasitic agentsPolyethylene glycolMicroparticle
The invention provides an oily injection containing antiparasitic agent / polyethylene glycol drug-loading microparticles which is prepared by suspending drug-loading microparticles, which are composed of antiparasitic agent and solid polyethylene glycol, in an oily medium. The antiparasitic agent comprises ivermectin, abamectin, eprinomectin, doramectin, milbemycin oxime, moxidectin, albendazole oxide or hydrochloride thereof, oxfendazole or hydrochloride thereof, closantel base or sodium salt thereof; polyethylene glycol with molecular weight of more than 6,000is preferably selected for preparation of the oily injection; and one of isopropyl myristate, injection soybean oil, maize oil and tea seed oil is preferably selected for preparation of the oily injection.
Owner:王玉万
Method for synthesizing milbemycin oxime compound
InactiveCN106565740AHigh yieldMild reaction conditionsOrganic chemistryBulk chemical productionChemical synthesisAvermectin B1a
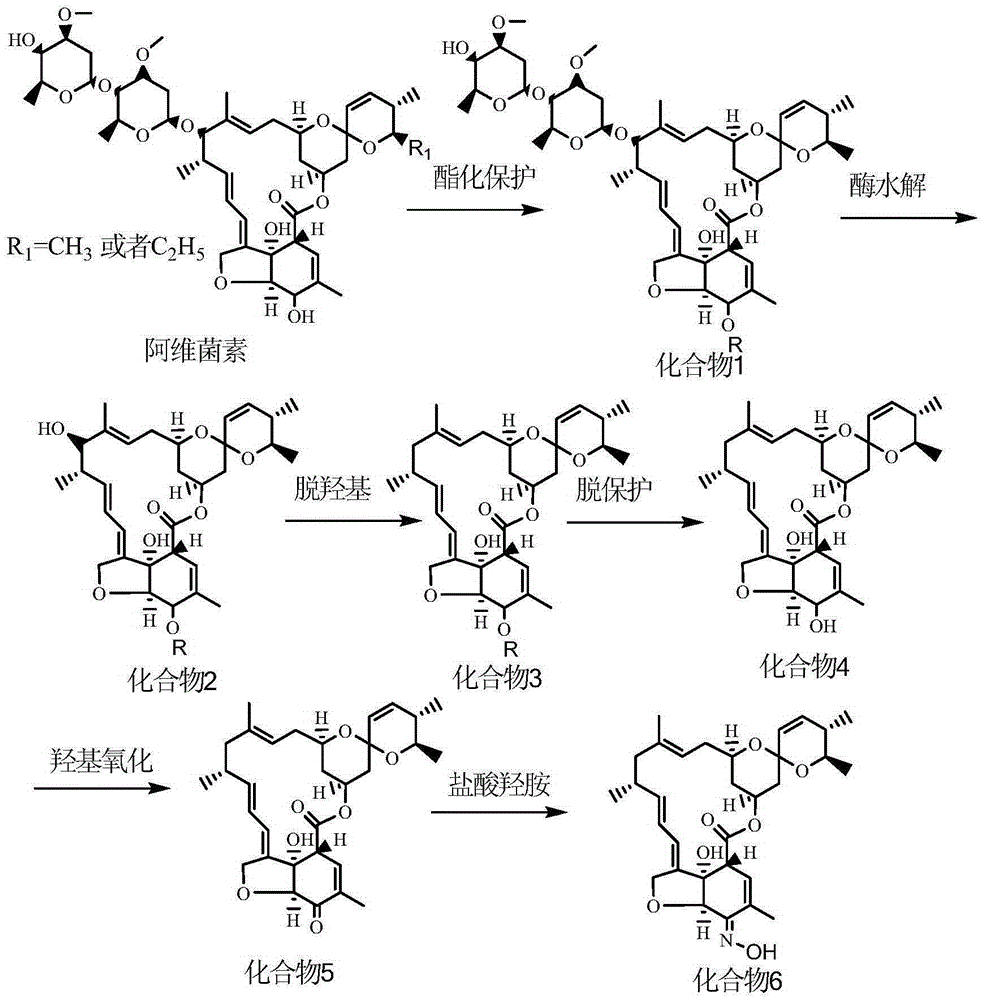
The object of the invention is to provide a method for synthesizing a milbemycin oxime drug. The implementation method provided by the invention comprises the steps: subjecting one or the mixture of avermectin B1a and avermectin B1b, which serves as a raw material, to a hydroxyl protecting reaction so as to protect a hydroxyl group at the C5 position; then, carrying out hydrolysis so as to remove a glycosyl group at the C13 position; then, carrying out a reaction so as to remove a hydroxyl group at the C13 position; and then, removing a hydroxyl protection group at the C5 position, carrying out hydroxyl oxidation so as to produce keto-carbonyl, and carrying out an oximation reaction, thereby finally obtaining a milbemycin oxime compound. According to the method, the milbemycin oxime compound product is synthesized from avermectins, which serve as the raw material, by a chemical synthesis method; and the chemical synthesis method has mature route and has no need of taking milbemycin, which is required to be obtained through fermentation, as the raw material, so that the cost and scarcity of the raw material are reduced, the large-scale industrial production is better facilitated, milbemycin oxime products are enriched, and thus, the shortage of domestic pet parasite treatment drugs is made up.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Preparation method of insect-repellent hairball remedy
InactiveCN106720943AIncrease gastrointestinal motilityImprove its digestibilityFood processingAnimal feeding stuffSickle alfalfaPraziquantel
The invention relates to an insect-repellent hairball remedy and a preparation method of the insect-repellent hairball remedy. The insect-repellent hairball remedy is prepared from the following raw materials in parts by weight: 10-15 parts of salmon bones, 5-10 parts of salmon scales, 5-10 parts of pteridium aquilinum, 5-10 parts of black fungus, 5-10 parts of psyllium seed husks powder, 20-30 parts of sickle alfalfa, 10-15 parts of olive oil, 10-15 parts of duck fat, 3-6 parts of bacillus subtilis, 1-1.5 parts of calcium lactate, 2-5 parts of malt polysaccharide, 2-5 parts of taurine, 5-8 parts of hickory epicarp, 2-2.5 parts of mirabilite, 2-2.5 parts of carrot, 0.5-1 part of milbemycin oxime and 0.3-0.5 part of praziquantel.
Owner:沈爱琴
A kind of method for preparing high-purity milbexime
The invention discloses a method for preparing high-purity milbemycin. After extracting milbemycin A3 and milbemycin A4 from fermentation broth and preliminarily purifying them, they are oxidized with an oxidizing agent with low oxidation activity and subjected to silica gel chromatography. Increase the content of milbemycin A3 ketone and milbemycin A4 ketone from less than 25% to more than 75%, and naturally precipitate crystals during the concentration process, making milbemycin A3 ketone and milbemycin A4 ketone The content of milbemycin A3 oxime and milbemycin A4 oxime is adjusted to be 1:4 by resin chromatography, and the content of the product can be improved to More than 98%. The whole process is relatively simple, requires less equipment, has good versatility, and is suitable for large-scale industrial production.
Owner:CHONGQING DAXIN PHARMA +2
Long-acting compound anthelmintic liquid preparation as well as preparation method and application thereof
ActiveCN112006981AHigh kill rateBroaden the Spectrum of Insect ResistanceOrganic active ingredientsPharmaceutical delivery mechanismAntiparasiticAntihelmintics
The invention relates to a long-acting compound anthelmintic liquid preparation as well as a preparation method and application thereof, and belongs to the field of veterinary drug preparations. The compound preparation is mainlycomposed of anti-parasitic active ingredients, namely milbemycin oxime, benzimidazoles and juvenile hormone ingredients, and comprises, in percent by mass, 0.5%-1% of milbemycin oxime, 5%-10% of benzimidazoles, 3%-5% of juvenile hormone, 0.1%-5% of macromolecule suspending agent, 0.1%-5% of wetting agent, 0.01%-1% of antioxidant and the balance of a dispersion medium.According to the invention, anti-parasitic drugs are combined for use, the insect-resistant spectrum is expanded, and a comprehensive and efficient in-vivo and in-vitro simultaneous-expelling effect is achieved.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Milbemycin oxime nano suspension for treating dog parasitic diseases and preparation method thereof
InactiveCN104208022AImprove performanceExtended stayOrganic active ingredientsSolution deliverySide effectSuspending Agents
The invention discloses a milbemycin oxime nano suspension for treating dog parasitic diseases and a preparation method thereof. Each 100 mL of suspension contains 2 to 5 grams of milbemycin oxime, 0.1 to 0.4 mL of wetting agent, 3.0 to 5.5 grams of suspending agent, 1.0 to 2.0 grams of flocculating agent, and the balance being purified water or injection water. The provided milbemycin oxime nano suspension is mainly made of milbemycin oxime, and at the same time, a wetting agent, a suspending agent, and a flocculating agent are added, so the milbemycin oxime nano suspension has a stable property and can be used to prepare oral or injection drugs. The detention time of the drugs in human body is prolonged, thus the drugs can be fully absorbed by human body, and the biological utilization degree is improved. The milbemycin oxime nano suspension is used to treat dog in-vitro / in-vivo parasitic diseases in clinic and has the advantages of safety, good curative effect, little side effect, and user-friendliness.
Owner:ZHENGZHOU HOUYI PHARMA
Antibody library of bacteriophages and applications in immunoassay of pesticide residue
InactiveCN101289760BWide range of affinity optionsIncrease screening throughputPeptide librariesMicroorganism librariesBiotechnologyPhage antibodies
The invention relates to a phage antibody library which assembles an ScFv gene fragment between Restriction Enzyme cutting sites of SfiI and NotI of a pCANTAB5E carrier. The phage antibody library is characterized in that the ScFv gene fragment can be affinitive and enriched with antigens of 16-membered macrolide agricultural chemicals to form a soluble single-chain antibody and the phage antibody library is applied to immunoassay of pesticide residue. The phage antibody library has the advantages that the antibody with high affinity can be obtained without animal immunization, the test period is short, the antibody library is the phage antibody library which takes small molecular milbemycin oxime of a 16-membered macrolide generic structure as immunogen to construct, the antibody librarytheoretically can directly obtain a specific antibody library of 16-membered macrolide compound through screening, the screening flux is high, the efficiency is high, the specificity is strong, and the affinity selecting range is wide, so that the phage antibody library has wide application prospect in the aspects such as agricultural chemical antibody preparation, testing technique development and the like.
Owner:JIANGSU ACAD OF AGRI SCI
Pharmaceutical composition for treating ear mites and preparation method of pharmaceutical composition
ActiveCN112641723AGood curative effectSolution stableOrganic active ingredientsSenses disorderOral medicineEar mite
The invention relates to the technical field of pharmaceutical compositions, in particular to a pharmaceutical composition for treating ear mites. The pharmaceutical composition is transparent milbemycin oxime ear drops, and comprises the following raw material medicines in percentages by weight: 0.1-1.0% of milbemycin oxime, 19.0-92.5% of a solvent, 2.0-50.0% of a solubilizer and 2.0-30.0% of a stabilizer. The ear drops provided by the invention are an innovative preparation for treating ear mites, can be dropped into ear canals of cats and dogs for use, directly act on ear mites, have an extremely strong curative effect on medium and severe ear mite infection, and are high in safety. The composition prepared by the invention has the characteristics of being convenient to administrate, accurate in dosage, lasting in effect and strong in curative effect, is non-irritant due to ear drop use, and has higher animal medicine taking compliance compared with oral medicines.
Owner:南京朗博特动物药业有限公司
A kind of preparation method of milbexime
ActiveCN105254644BIncrease production capacityLow costOrganic chemistryHydroxylamine HydrochlorideKetone
The invention provides a preparation method of milbemycin oxime. The preparation method of milbemycin oxime comprises the following steps: extracting milbemycins, namely taking milbe mycelia obtained through fermentation as a raw material, extracting, concentrating, extracting, concentrating, extracting again and concentrating again, so as to obtain milbemycin; preparing milbemycin ketone, namely taking the milbemycin as a raw material, establishing an oxidation reaction system, after reaction is finished, filtering, concentrating, extracting and concentrating the reaction system, so as to obtain an intermediate product milbemycin ketone; synthesizing milbemycin oxime, namely dissolving milbemycin ketone with methanol and dioxane, dropwise adding hydroxylamine hydrochloride solution and reacting, concentrating the reaction system, extracting, drying and concentrating, so as to obtain a milbemycin oxime crude product; and purifying milbemycin oxime, namely crystallizing the milbemycin oxime crude product with a mixed solvent of trichloromethane and normal heptane, dissolving a crystalline product with ethanol, dropwise adding into water while stirring for carrying out crystallization, filtering, and drying, so that the milbemycin oxime finished product is obtained. The preparation method of the milbemycin oxime has the advantages that productivity is greatly improved, and cost is obviously reduced, so that the preparation method provided by the invention is obviously better than an existing preparation method.
Owner:HUBEI HONCH PHARMA
Method for recovering manganese dioxide from milbemycin oxime filter residues
ActiveCN112299488AOxidative activityEfficient recyclingManganese oxides/hydroxidesProcess efficiency improvementCombustible gasManganese oxide
The invention discloses a method for recovering manganese dioxide from milbemycin oxime filter residues, which belongs to the technical field of manganese dioxide recovery and sequentially uses ethanol and deionized water to perform top washing on the manganese dioxide filter residues. According to the method, manganese dioxide can be effectively recycled from the filter residues generated in milbemycin oxime production through a manganese dioxide oxidation method, acetone and residual products in the filter residues can be effectively removed, the manganese dioxide is stopped from continuingto react with the products, combustible gas in the filter residues is removed, and potential safety hazards caused by reaction heat release are thoroughly avoided; meanwhile, the obtained manganese dioxide still has certain oxidation activity and can be reutilized after being recycled and treated, the production cost of enterprises is effectively reduced, the used ethyl alcohol and deionized waterare used for top washing, the raw materials are easy to obtain and low in price, the ethyl alcohol and the deionized water are non-toxic, and secondary pollution cannot be caused.
Owner:LIVZON GROUP FUZHOU FUXING PHARMACEUTICAL CO LTD
Giant panda epidermis repellent spray and preparation method thereof
InactiveCN108159192BImprove permeabilityAvoid weakening the efficacy of the drugOrganic active ingredientsAnthropod material medical ingredientsButanedioic acidPolythylene glycol
The invention discloses a giant panda epidermal insect repellent spray and a preparation method thereof. The spray is made of the following ingredients in parts by weight: 18-25 parts of minoxidil, 10-20 parts of stearic acid, and polyethylene glycol. 8-13 parts, Milbe oxime 22-34 parts, tea 8-15 parts, ivory shavings 7-18 parts, neem bark 11-21 parts, white tinea skin 10-16 parts, seaweed 3-6 parts, cocklebur 3-5 parts of Chinese mugwort, 2-6 parts of mugwort leaves, 4-7 parts of honeycomb, 8-16 parts of gallnuts, 2-3 parts of sodium diisooctyl sulfonate succinate, and 1-2 parts of fatty alcohol polyoxyethylene ether. The spray can quickly repel insects and relieve itching, has a long-lasting effect and is not easy to relapse. At the same time, it is non-toxic to giant pandas and has no side effects.
Owner:SICHUAN AGRI UNIV +1
A kind of streptomyces producing 5-ketomilbemycin and the method for producing 5-ketomilbemycin
The invention relates to the technical field of gene recombination, and particularly relates to streptomycete generating 5-keto-milbemycins and a method for producing 5-keto-milbemycins. The invention provides a recombinant vector for knocking out the milF gene of streptomycete, including the homologous fragment of the milF gene, wherein the sequence of the fragment is obtained by losing 105-767 nucleotides from the nucleotides from the sites 158-924 in the nucleotide sequence shown by SEQ ID No.2. The invention also provides an establishment method of the recombinant vector, a method for knocking out the milF gene in streptomycete by use of the recombinant vector and streptomycete without milF gene. The streptomycete can be directly fermented to generate 5-keto-milbemycins, so that the synthesis process of milbemycin oxime is simplified, and the pollution caused by the traditional chemical synthesis method is avoided.
Owner:ZHEJIANG HISUN PHARMA CO LTD
A kind of separation and purification method of milbexime
The invention provides a milbemycin oxime separation and purification method. The milbemycin oxime separation and purification method comprises the following steps: carrying out crude separation on a sample to be separated through silicagel column chromatography, wherein the silicagel column chromatography adopts wet-process column packing and dry-method sample loading; purifying the product after crude separation by high performance liquid chromatography; concentrating the purified sample by a nanofiltration membrane to obtain concentrated solution; performing reduced-pressure vaporization to the concentrated solution, filtering and drying to obtain a refined product; dissolving the refined product, heating up, dropwise adding isooctyl alcohol, normal heptane or petroleum ether, cooling to be crystallized, and filtering to obtain a crystallized product; dissolving the crystallized product, filtering, dropwise adding filtrate into purified water stirred constantly, after dropwise adding, carrying out suction filtration to obtain a crystal transformation product; drying the crystal transformation product, crushing, and drying again to obtain milbemycin oxime finished product. The method can realize industrial production of milbemycin oxime in China firstly, and the product has high purity and high yield which are higher than those of the similar products at home and abroad.
Owner:HUBEI HONCH PHARMA
Preparation method of milbemycin oxime
Owner:LIVZON GROUP FUZHOU FUXING PHARMACEUTICAL CO LTD
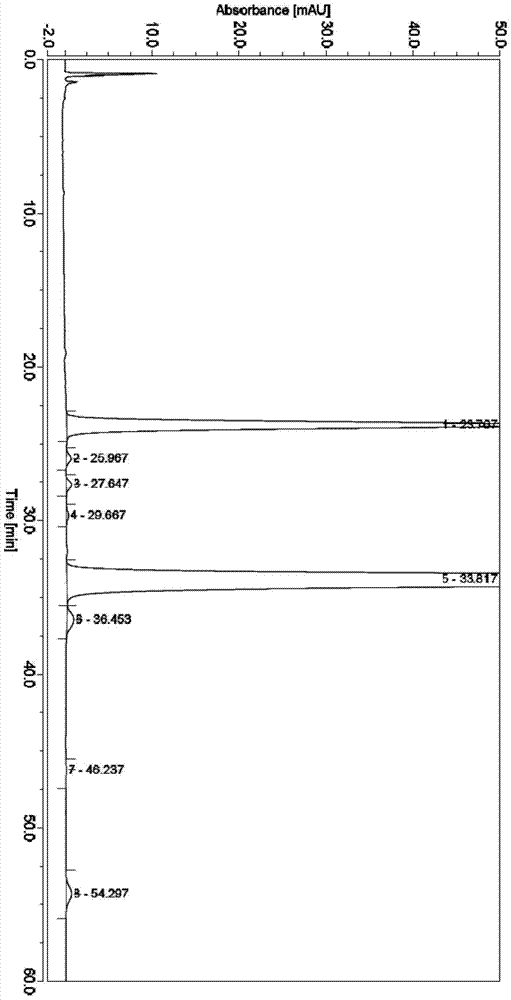
Method for simultaneously detecting contents of praziquantel, clofenoxine and milbemycin oxime
ActiveCN114280200AThe detection method is simpleShorten the timeComponent separationAgainst vector-borne diseasesO-Phosphoric AcidGradient elution
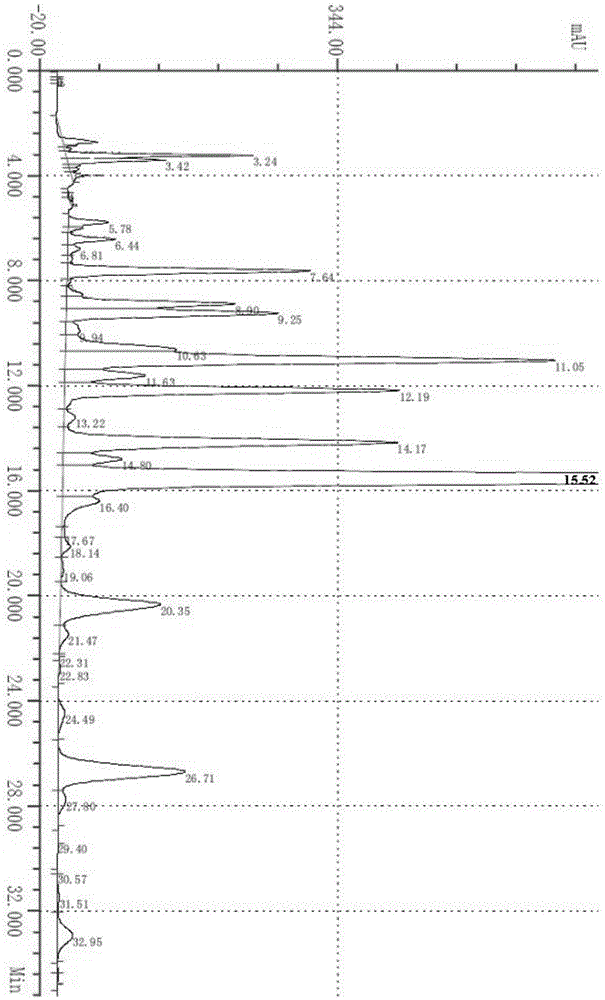
The invention provides a method for simultaneously detecting contents of praziquantel, clofenoxine and milbemycin oxime, which comprises the following steps: firstly preparing a blank solution, then preparing a test solution containing praziquantel, clofenoxine and milbemycin oxime, finally preparing a system applicability test solution, respectively injecting into a liquid chromatograph, and recording chromatograms under ultraviolet wavelength; according to the method, acetonitrile is taken as a diluent, octadecylsilane chemically bonded silica is taken as a chromatographic column in a liquid chromatograph, gradient elution is carried out, the flow rate is 1.0 mL / min, the column temperature is 30 DEG C, the ultraviolet detection wavelength is 230 nm, and the sample size is 20 mu L; the mobile phase A is a phosphoric acid solution with the content of 10-30 wt%, and the mobile phase B is acetonitrile with the content of 70-90 wt%; the detection method provided by the invention is simple and convenient, saves time, reduces cost, has good specificity, accuracy, linearity, durability and precision, and is suitable and accurate for detecting the content of the three components, so that the detection method can be used for daily detection of the pet medicine.
Owner:上海汉维生物医药科技有限公司
A kind of purification method of milbemycin oxime crude product
The invention relates to a purification method of a milbemycin oxime crude product. The method includes the process steps that firstly, the milbemycin oxime crude product is washed at the pH of 8-9; then, a milbemycin oxime wet product obtained after filtering is dissolved in butyl acetate or isobutyl acetate and then decolored with macroporous resin, water is added for mixing, standing layering and filtering are carried out, obtained filter liquor is dried with anhydrous calcium chloride, filter liquor is collected after filtering, water is added again for mixing, filtering and drying are carried out after heating crystallization, and a milbemycin oxime pure product can be obtained. By means of the method, impurities in the milbemycin oxime crude product are effectively separated, the quality of the finished product is improved, and the home and abroad market competiveness of the product is easily enhanced.
Owner:宁夏泰瑞制药股份有限公司
Panda epidermis insect repellent spray and preparation method thereof
InactiveCN108159192AImprove permeabilityAvoid weakening the efficacy of the drugOrganic active ingredientsAnthropod material medical ingredientsSide effectCuticle
The invention discloses a panda epidermis insect repellent spray and a preparation method thereof. The spray is prepared from the following components in parts by weight: 18 to 25 parts of minoxidil,10 to 20 parts of stearic acid, 8 to 13 parts of polyethylene glycol, 22 to 34 parts of milbemycin oxime, 8 to 15 parts of tea leaves, 7 to 18 parts of ivory crumbs, 11 to 21 parts of bead tree bark,10 to 16 parts of cotex dictamni, 3 to 6 parts of seaweed, 3 to 5 parts of fructus xanthii, 2 to 6 parts of folium artemisiae argyi, 4 to 7 parts of honeycomb, 8 to 16 parts of galla chinensis, 2 to 3parts of dioctyl sulfosuccinate sodium salt, and 1 to 2 parts of fatty alcohol-polyoxyethylene ether. The spray can be used for quickly expelling insects and relieving itching, is durable in effect and not easy to recur, and has no toxic and side effects on pandas at the same time.
Owner:SICHUAN AGRI UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com