Milbemycin oxime and praziquantel flavored tablet and preparation method thereof
A technology of mirboxime and praziquantel, which can be applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. and other problems to achieve the effect of improving stability, increasing active feeding rate, and increasing willingness to feed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
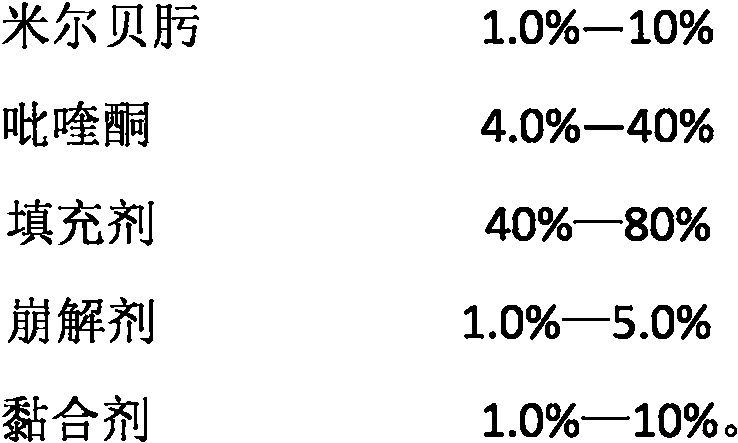
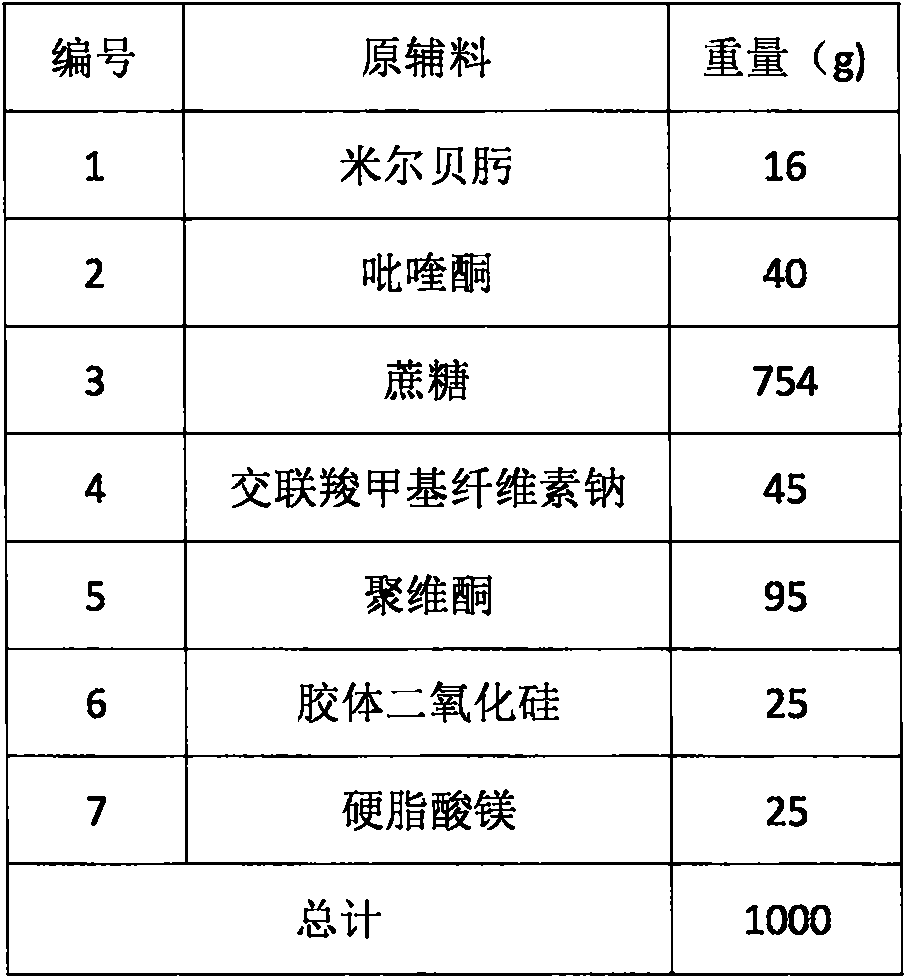
[0045] Tablet prescription:
[0046] Table 1
[0047]
[0048] Coating Solution Prescription:
[0049] Table 2
[0050]
[0051]
[0052] Note: Purified water will be dried during the coating preparation process, and the amount of purified water will not be calculated in the coating prescription.
[0053] Preparation:
[0054] 1) Pass the prescribed amount of milbexime, praziquantel, filler sucrose, and disintegrant croscarmellose sodium through a 50-mesh sieve, and put them into a fluidized bed, and the drugs and auxiliary materials are placed in the lower part of the fluidized bed Under the action of hot air flow at 45°C, mix evenly to obtain a mixture;
[0055] 2) Spray into the mixture in step 1) the adhesive agent 15% povidone aqueous solution of recipe quantity, the powder that has absorbed the solution begins to bond into small particles, along with the continuous adding of adhesive agent povidone aqueous solution, small particle The particles slowly aggre...
Embodiment 2
[0065] Tablet prescription:
[0066] Table 4
[0067]
[0068] Coating Solution Prescription:
[0069] table 5
[0070]
[0071] The preparation method is the same as in Example 1, wherein the weight gain of the coating treatment is 3%.
[0072] Note: Purified water will be dried during the coating preparation process, and the amount of purified water will not be calculated in the coating prescription.
Embodiment 3
[0074] Tablet prescription:
[0075] Table 6
[0076]
[0077]
[0078] Coating Solution Prescription:
[0079] Table 7
[0080]
[0081] Note: Purified water will be dried during the coating preparation process, and the amount of purified water will not be calculated in the coating prescription.
[0082] The preparation method is the same as in Example 1, wherein the weight gain of the coating treatment is 3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com