Milbemycin oxime separation and purification method
A milbexime, separation and purification technology, applied in the direction of organic chemistry, etc., to reduce environmental pollution, increase production capacity, and reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
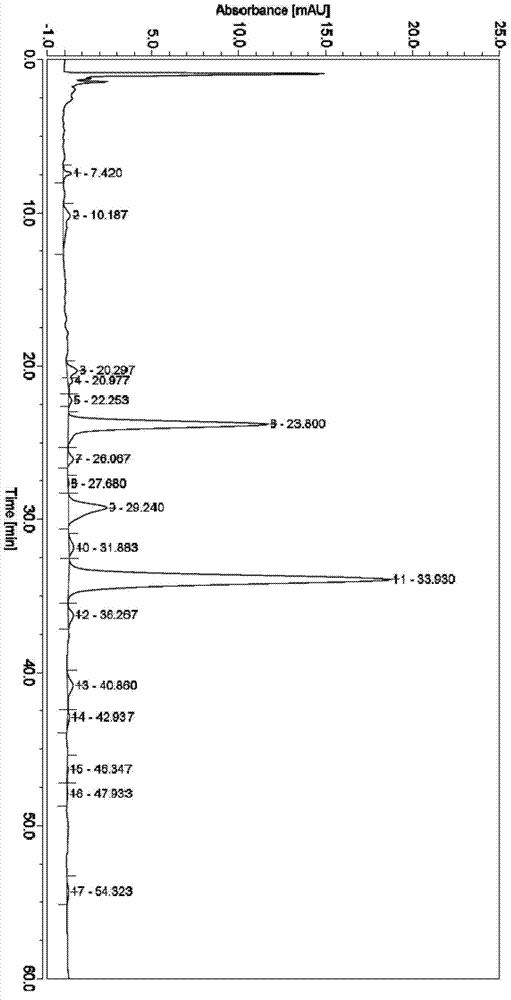
[0028] (1), take by weighing 120g milbexime crude product, the liquid chromatography detection spectrogram of crude product is as follows figure 1 Shown, where milbexime A 4 Calibration content is 8.4%, milbexime A 3 The calibration content of milbexime is 5.6%. Dissolve the crude product of milbexime with 110g of acetone, then add 120g of silica gel and mix well, place it in a vacuum oven and dry it under vacuum at 35-45°C for 4-10 hours; then weigh 240g of silica gel for column chromatography , soak the silica gel with 0.8 to 2.5 times the volume of chloroform and stir evenly, then pour it into a Ф6.8cm×100cm glass chromatography column, beat evenly, and elute with 1500mL n-heptane to balance; then the dried mixture is washed with 300mL n-heptane Mix the heptane thoroughly, load it on the column, and the loading volume is 5% to 20% of the column volume;
[0029] Adopt the mixed solvent of n-heptane / acetone to carry out elution as mobile phase, the volume ratio of n-heptane...
Embodiment 2
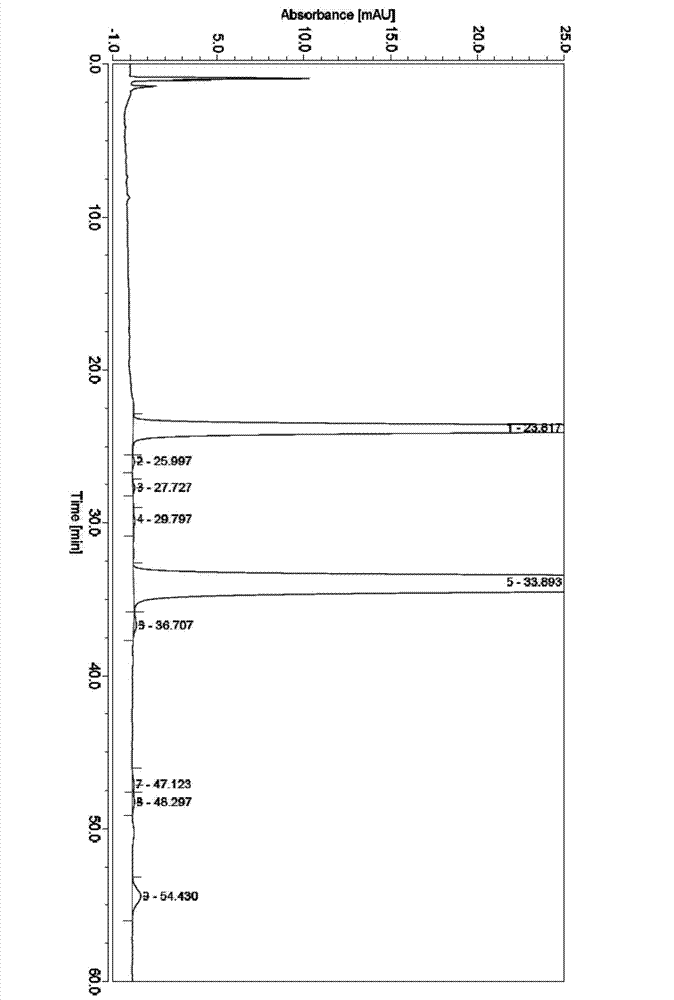
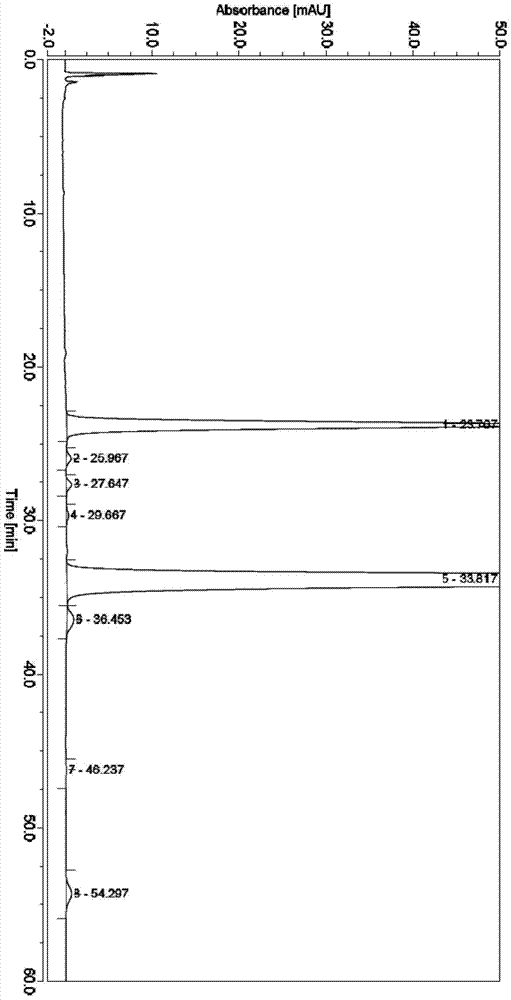
[0039] (1), weigh 3Kg crude product of milbe oxime, wherein milbe oxime A 4 Calibration content is 8.7%, milbexime A 3 The calibration content of milbexime is 6.5%. Dissolve the crude product of milbexime with 3.11Kg of acetone, then add 3Kg of silica gel and mix well, place it in a vacuum oven and dry it under vacuum at 40°C for 6h; then weigh 6Kg of silica gel for column chromatography, and Soak in 12L chloroform and stir evenly, then pour it into a Ф22.8cm×100cm glass chromatography column, beat evenly, and elute with 40L n-heptane to balance; then mix the dried mixture thoroughly with 7.5L n-heptane, and load it on the column On, the sample volume is 5% to 20% of the column volume;
[0040] Adopt the mixed solvent of n-heptane / acetone to carry out elution as mobile phase, the volume ratio of n-heptane / acetone is 97:3, HPLC monitors the elution situation of milbexime, collects milbexime A 3 and A 4 Components with a total purity greater than 40% were evaporated to drynes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com