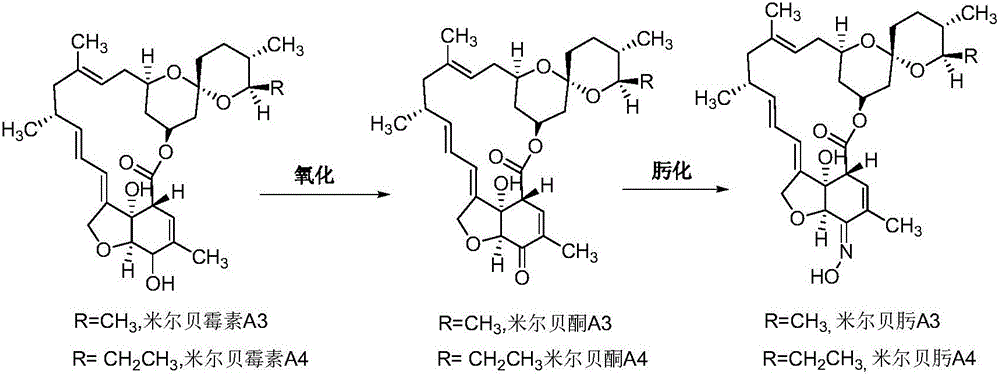
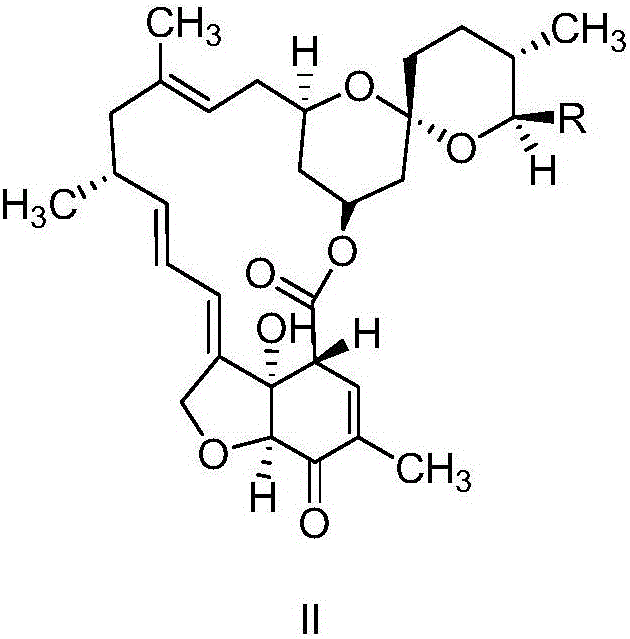
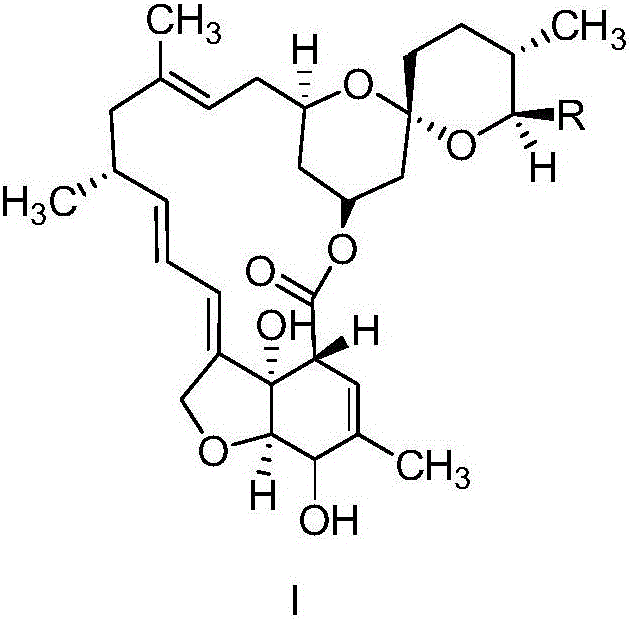
Preparation method of milbemycin oxime intermediate
A technology of milbexime and intermediates, which is applied in the field of preparation of milbexime intermediates, can solve problems such as difficult separation and extraction, low yield, and many by-products, achieve mild reaction conditions, reduce production costs, and yield and The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh 5g of milbemycin A4 and put it into a 250mL four-necked bottle, add 25ml of acetonitrile, add 0.73g of 2,2,6,6-tetramethylpiperidine-nitrogen-oxide (tempo) and 222mg of potassium bromide, Stir while maintaining the temperature at 20-25°C. Next, 62 mL of sodium hypochlorite aqueous solution with a pH of 9.0 was added under stirring conditions, wherein the concentration of sodium hypochlorite in the solution was 0.3 mol / L, and the addition was completed within 45 minutes, and then incubated at 20-25°C for 30 minutes. Then add 30mL of 5% (w / v) sodium thiosulfate solution to quench the reaction, concentrate acetonitrile at 40°C until there is no dripping, add 100mL of dichloromethane to stir, separate layers, add 50mL of dichloromethane to the aqueous phase for extraction, and combine the obtained The organic phase was washed once with 75mL of 5% (w / v) NaCl solution, and the organic phase obtained after washing was directly concentrated to dryness to obtain 5.8g of so...
Embodiment 2
[0038] Weigh 5g of milbemycin A3 / A4 (mass content 90%, wherein A3:A4=1:4) into a 250mL four-necked bottle, add 25ml of ethyl acetate, add 2,2,6,6-tetramethyl Base piperidine-nitrogen-oxide (tempo) 0.14g and potassium bromide 222mg, keep stirring at -2-5°C. Next, add 41 mL of sodium hypochlorite aqueous solution with a pH of 11.5 under stirring conditions, wherein the concentration of sodium hypochlorite in the solution is 0.5 mol / L, the addition is completed within 15 minutes, and then the reaction is incubated at -2-5°C for 30 minutes. Then add 30mL 5% (w / v) sodium thiosulfate solution to quench the reaction, add 75mL ethyl acetate and stir, separate layers, add 50mL ethyl acetate to the aqueous phase for extraction, combine the organic phases obtained and use 75mL 5% (w / v) v) NaCl solution was washed once, and the organic phase obtained after washing was directly concentrated to dryness to obtain 5.6 g of solids with a yield of 82%, wherein the oxidation product Milbetone A3...
Embodiment 3
[0040] Weigh 5g of Milbemycin A3 / A4 (mass content 90%, wherein A3:A4=1:4) into a 250mL four-necked bottle, add 25ml of dichloromethane, add 2,2,6,6-tetramethyl Base piperidine-nitrogen-oxide (tempo) 0.014g and potassium bromide 2220mg, keep stirring at 0-5°C. Next, add 47mL of sodium hypochlorite aqueous solution with a pH of 10.5 under stirring conditions, wherein the concentration of sodium hypochlorite in the solution is 0.8mol / L, the addition is completed within 20min, and then the reaction is incubated at 0-5°C for 30min. Then add 30mL 5% (w / v) sodium thiosulfate solution to quench the reaction, add 75mL dichloromethane to stir, separate layers, add 50mL dichloromethane to the aqueous phase for extraction, combine the organic phases obtained and use 75mL 5% (w / v) v) NaCl solution was washed once, and the organic phase obtained after washing was directly concentrated to dryness to obtain 5.3 g of solids with a yield of 78%, wherein the oxidation product Milbetone A3 was 16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com