Biological catalysis system for oxidizing phenolic compound and application thereof
A technology of phenolic compounds and catalytic systems, applied in the field of biocatalytic systems, can solve problems such as insufficient mild conditions, pollution, and poor stereoselectivity, and achieve the effects of safe reaction production, simple preparation, and mature technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Construction of CreH, CreI, CreJ, CreE, CreF, CreD, CreG and CreC enzyme expression strains.
[0056] First, according to the corresponding coding genes creH (Ncgl0528), creI (Ncgl0529), creJ (Ncgl0530), creE (Ncgl0525), creF (Ncgl0526), The sequence design primers of creD (Ncgl0524), creG (Ncgl0527) and creC (Ncgl0523) are as follows:
[0057] creH-F: GCGCCATATGGCTAATAAATCTTTTCCCCAAGCCC
[0058] creH-R: ACGCGGATCCGTGGACTAGGCATGTGTATC
[0059] creI-F: TCGCCATATGGGAGACACCATGACCAACAGT
[0060] creI-R: GCCCAAGCTTAACGAGGTAGTACGGGTACA
[0061] creJ-F: CGTGCCATGGGCACAATGACTTCCCAGACT
[0062] creJ-R: CGGGAAGCTTAGCGTTCCAAGTCACGGGAA
[0063] creE-F: GACACATATGAATACTTCAGCTGAAACTGGA
[0064] creE-R: GGAGCTCTCCCAAGCGGGTAAAT
[0065] creF-F: GCGCCATATGAAGATCATGTCTACTATTCATT
[0066]creF-R: TCATAAGCTTTCACACTTGCGTTTCTGGCG
[0067] creD-F: GACACATATGACTCGCAGTAATTTACCCGC
[0068] creD-R: CGGAATTCGAGAAGCACGCCTGGTTG
[0069] creG-F: GACACATATGCCTAGTCCACGCACTGTTC
[0070] creG-...
Embodiment 2
[0075] Protein expression and purification of CreH, CreI, CreJ, CreE, CreF, CreD, CreG and CreC.
[0076] The expression strains obtained above were respectively inoculated in LB containing 50 ug / mL kanamycin at 37° C., 220 rpm, and cultured overnight. Inoculate fresh LB medium (containing 4% glycerol, 50 μg / mL kanamycin) at 1:100 (v / v), culture at 37°C, 220 rpm for 2-4 hours, until OD 600 = 0.4-0.6. Add IPTG with a final concentration of 0.2mM, and culture at 20°C and 220rpm for 18 hours. 4°C, centrifuge at 5000rpm for 5min to collect the cells, and use 50mL of lysis buffer (NaH 2 PO 4 50mM, NaCl 300mM, glycerol 10%, imidazole 10mM) dissolved and mixed with a vortex shaker. The bacteria were ultrasonically lysed with an ultrasonic instrument, the lysate was collected separately, centrifuged at 12000g for 30min at 4°C, and the supernatant was taken. Add 1mL Ni-NTA resin to every 50mL supernatant, and incubate at 4°C for 20min. Then add it to the protein purification colu...
Embodiment 3
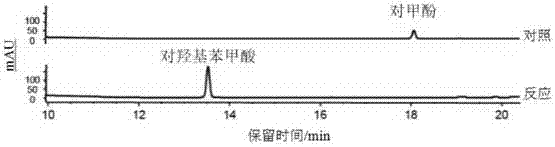
[0079] Phosphorylation of p-cresol (4-methylphenol).
[0080] Establish an enzyme reaction system:
[0081] p-cresol 1mM,
[0082] Mg 2+ 20mM,
[0083] mn 2+ 2mM,
[0084] ATP 1mM,
[0085] CreH protein 10 μM,
[0086] CreI protein 10 μM.
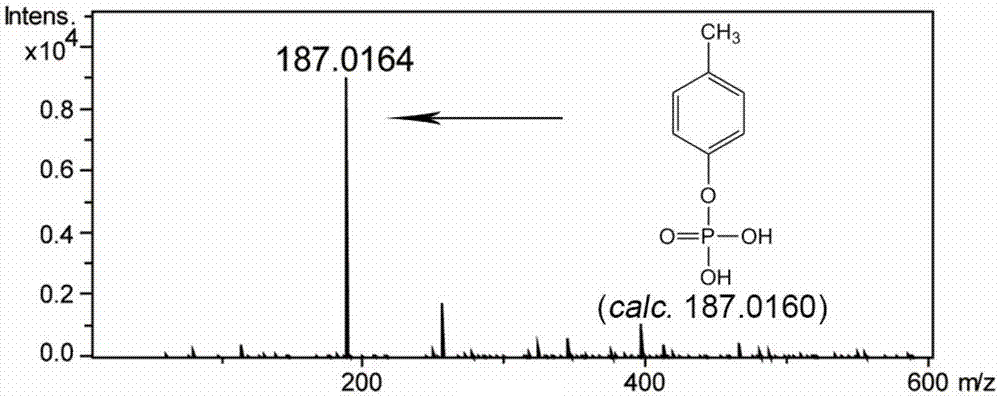
[0087] After incubation at 30°C for 30 minutes, p-cresol can be converted into p-cresol phosphorylation product, and the conversion rate is 100%. The reaction formula is as follows:
[0088]
[0089] High-resolution mass spectrometry of p-cresol phosphorylation products see figure 1 , NMR spectrum see figure 2 .
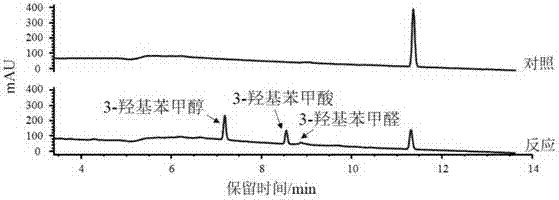
[0090] Among them, CreH and CreI proteins can be replaced by their homologous proteins or functional equivalents, and p-cresol can also be converted into p-cresol phosphorylation products.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com